Abstract
Purpose
The patient impact of chemotherapy-induced alopecia (CIA) is high. Scalp cooling is applied to reduce CIA. The potential optimum post-infusion cooling times (PICTs) are currently unknown.
Methods
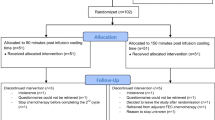
Scalp cooling was applied in 53 patients receiving docetaxel chemotherapy with 90-min PICT (observational part). Also 15 non-scalp-cooled patients were included. If hair preservation was observed in >80 % of the patients, randomisation between 45 and 90-min PICT was planned. Patients reported tolerance of scalp cooling and use of head covering.
Results
Observational study: 81 % of scalp-cooled patients did not require head covering versus 27 % of non-scalp-cooled patients. Randomised study: 79 % of 38 patients with 90-min PICT did not need head covering versus 95 % of 38 patients with 45-min PICT (p = 0.04). Scalp cooling was very well tolerated (visual analogue scale = 79).
Conclusion
A 45-min PICT can be recommended in 3-weekly docetaxel regimens with a dose of 75 or 100 mg/m2, administered in 60 min. The shorter PICT is a major advantage in time investment for patients. Patients (women and men) who receive docetaxel, except combined with doxorubicin and cyclophosphamide (taxotere, adriamycin and cyclophosphamide (TAC)) should be informed about the protective effect and high tolerability of scalp cooling in avoiding CIA.
Similar content being viewed by others
References
Grevelman EG, Breed WP (2005) Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol 16:352–358
Breed WPM, van den Hurk CJG, Peerbooms M (2011) Presentation, impact and prevention of chemotherapy-induced hair loss; scalp cooling potentials and limitations. Expert Rev Dermatol 6:109–125
Christodoulou C, Klouvas G, Efstathiou E et al (2002) Effectiveness of the MSC cold cap system in the prevention of chemotherapy-induced alopecia. Oncology 62:97–102
Katsimbri P, Bamias A, Pavlidis N (2000) Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer 36:766–771
Lemenager M, Lecomte S, Bonneterre ME et al (1997) Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer 33:297–300
Lundgren-Eriksson L, Edbom G, Olofsson Y et al (1999) Total prevention of taxoid-induced alopecia by a new model of cold cap (dignitana). Eur J Cancer 35(suppl 4):376
Auvinen PK, Mahonen UA, Soininen KM et al (2010) The effectiveness of a scalp cooling cap in preventing chemotherapy-induced alopecia. Tumori 96:271–275
Ridderheim M, Bjurberg M, Gustavsson A (2003) Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer 11:371–377
ElGenidi M (2001) Prevention of chemotherapy-induced alopecia by the new digital scalp cooler device. Eur J Cancer 37(Suppl 6):357
Massey CS (2004) A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs 8:121–130
Cortes JE, Pazdur R (1995) Docetaxel. J Clin Oncol 13:2643–2655
Middleton J, Franks D, Buchanan RB et al (1985) Failure of scalp hypothermia to prevent hair loss when cyclophosphamide is added to doxorubicin and vincristine. Cancer Treat Rep 69:373–375
Hilton S, Hunt K, Emslie C et al (2008) Have men been overlooked? A comparison of young men and women’s experiences of chemotherapy-induced alopecia. Psycho-Oncology 17:577–583
Baxley KO, Erdman LK, Henry EB, Roof BJ (1984) Alopecia: effect on cancer patients’ body image. Cancer Nurs 7:499–503
de Boer-Dennert M, de Wit R, Schmitz PI et al (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Tabernero J, Climent MA, Lluch A et al (2004) A multicentre, randomised phase II study of weekly or 3-weekly docetaxel in patients with metastatic breast cancer. Ann Oncol 15:1358–1365
Ravdin PM, Burris HA 3rd, Cook G et al (1995) Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol 13:2879–2885
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
O’Shaughnessy J, Miles D, Vukelja S et al (2002) Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 20:2812–2823
Sjostrom J, Blomqvist C, Mouridsen H et al (1999) Docetaxel compared with sequential methotrexate and 5-fluorouracil in patients with advanced breast cancer after anthracycline failure: a randomised phase III study with crossover on progression by the Scandinavian Breast Group. Eur J Cancer 35:1194–1201
Chan S, Friedrichs K, Noel D et al (1999) Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17:2341–2354
Chevallier B, Fumoleau P, Kerbrat P et al (1995) Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol 13:314–322
Bonneterre J, Roche H, Monnier A et al (2002) Docetaxel vs 5-fluorouracil plus vinorelbine in metastatic breast cancer after anthracycline therapy failure. Br J Cancer 87:1210–1215
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Fossella F, Pereira JR, von Pawel J et al (2003) Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 21:3016–3024
Chang HR, Glaspy J, Allison MA et al (2010) Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer 116:4227–4237
van den Hurk CJ, Peerbooms M, van de Poll-Franse LV, Nortier JW, Coebergh JW, Breed WP (2012) Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients - Results of the Dutch Scalp Cooling Registry. Acta Oncol 51(4):497–504
Acknowledgments
Special thanks go to all patients who participated in the study, to A. Willemse who assisted in data collection, to M. Peerbooms who assisted in data entry and to W. Peters, MD., of Catharina Hospital Eindhoven, who was willing to function as an independent advisor and to answer patients’ questions. Furthermore, we would like to thank the nurses and doctors who performed the study in the following hospitals: Medisch Centrum Alkmaar, Alkmaar; Tergooiziekenhuizen, Blaricum; Máxima Medisch Centrum, Eindhoven; Elkerliek, Helmond; Jeroen Bosch, ’s-Hertogenbosch; Leids Universitair Medisch Centrum, Leiden; St. Antonius, Nieuwegein; St. Elisabeth, Tilburg; Twee Steden, Tilburg; and Diaconessen, Utrecht. This work was supported by Sanofi Aventis.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van den Hurk, C.J.G., Breed, W.P.M. & Nortier, J.W.R. Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer 20, 3255–3260 (2012). https://doi.org/10.1007/s00520-012-1465-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1465-0