Abstract
Goals of work
To assess the efficacy of adding aprepitant to a 5-HT3 antagonist and dexamethasone as salvage antiemetic therapy for breast cancer patients receiving their initial cycle of an anthracycline and cyclophosphamide (AC) and failing to achieve complete control of emesis.
Materials and methods
Eligibility: breast cancer patients receiving their first cycle of AC. Treatment: standard dose of a 5-HT3 antagonist and dexamethasone 8–10 mg IV/PO on day 1 prior to cycle 1 of AC and dexamethasone 4 mg bid on days 2 and 3. Patients without complete control (no emesis, no nausea, or rescue antiemetics) during cycle 1 could proceed to cycle 2. During cycle 2, patients received AC and identical antiemetics (except dexamethasone 4 mg qd on days 2 and 3) plus aprepitant 125 mg PO day 1 and 80 mg PO days 2 and 3. Primary endpoint: complete control, 0–120 h after chemotherapy.
Results
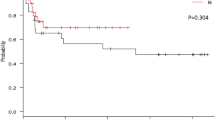
Sixty-two patients received cycle 1 of AC. Complete control cycle 1: 13 patients (21%; 95%CI, 12–33%). Of the 49 patients eligible for cycle 2, four elected not to continue on study. Of the 45 patients receiving cycle 2, 44 were evaluable. Complete control and complete response (no emesis, no rescue) for the 5-day study period improved from 0% to 18% (p = 0.14) and 7% to 36% (p = 0.02) on cycles 1 and 2, respectively.
Conclusions
In breast cancer patients receiving AC, the addition of aprepitant to a 5-HT3 antagonist and dexamethasone during cycle 2 of treatment improved antiemetic outcome. Although the improvement in the primary endpoint of complete control during cycle 2 was not significant, all secondary endpoints such as complete response and no emesis rates were significantly better during cycle 2. The extent of antiemetic control during cycle 2 was numerically inferior to the results achieved in a phase III trial employing aprepitant with cycle 1 of AC chemotherapy, suggesting that the preferred approach is to include aprepitant with the initial cycle of AC chemotherapy.




Similar content being viewed by others
References
de Boer-Dennert M, de Wit R, Schmitz PI et al (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Hickok JT, Roscoe JA, Morrow GR et al (2003) Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer 97:2880–2886 doi:10.1002/cncr.11408
Pollera CF, Giannarelli D (1989) Prognostic factors influencing cisplatin-induced emesis: definition and validation of a predictive logistic model. Cancer 64:1117–1122 doi:10.1002/1097-0142(19890901)64:5<1117::AID-CNCR2820640525>3.0.CO;2-R
du Bois A, Meerpohl HG, Vach W et al (1992) Course, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetron. Eur J Cancer 28:450–457 doi:10.1016/S0959-8049(05)80075-9
Hesketh P, Navari R, Grote T et al (1996) Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. J Clin Oncol 14:2242–2249
Hesketh PJ, Kris MG, Grunberg SM et al (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
DiBenedetto J Jr, Cubeddu LX, Ryan T et al (1995) Ondansetron for nausea and vomiting associated with moderately emetogenic cancer chemotherapy. Clin Ther 17:1091–1098 doi:10.1016/0149-2918(95)80087-5
Gralla RJ, Osoba D, Kris MG et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 17:2971–2994
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21:4112–4119 doi:10.1200/JCO.2003.01.095
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD et al (2003) Addition of the neurokinin-1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy induced nausea and vomiting: results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098 doi:10.1002/cncr.11433
Warr DG, Hesketh PJ, Gralla RJ et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23:2822–2830, (Erratum, J Clin Oncol 2005; 23:5851) doi:10.1200/JCO.2005.09.050
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494 doi:10.1056/NEJMra0706547
Perez EA (1999) 5-HT3 antiemetic therapy for patients with breast cancer. Breast Cancer Res Treat 57:207–214 doi:10.1023/A:1006233802863
Beck TM, Ciociola AA, Jones SE et al (1993) Efficacy of oral ondansetron in the prevention of emesis in outpatients receiving cyclophosphamide-based chemotherapy. The Ondansetron Study Group. Ann Intern Med 118:407–413
Hesketh PJ (1994) Treatment of chemotherapy-induced emesis in the 1990s: impact of the 5-HT3 receptor antagonists. Support Care Cancer 2:286–292 doi:10.1007/BF00365579
Hesketh PJ (2000) Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest 18:163–173 doi:10.3109/07357900009038248
Roila F, Hesketh PJ, Herrstedt J et al (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17:20–28 doi:10.1093/annonc/mdj936
Kris MG, Hesketh PJ, Somerfield MR et al (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947, (Erratum, J Clin Oncol 2006; 24:5341–5342) doi:10.1200/JCO.2006.06.9591
Ettinger DS, Bierman PJ, Bradbury B et al (2007) Antiemesis. J Natl Compr Canc Netw 5:12–33
Herrstedt J (2007) Chemotherapy-induced nausea and vomiting: ESMO clinical recommendations for prophylaxis. Ann Oncol 18(Suppl 2):ii83–ii85 doi:10.1093/annonc/mdm050
Vermeulen LC Jr, Matuszewski KA, Ratko TA et al (1994) Evaluation of ondansetron prescribing in US academic medical centers. Arch Intern Med 154:1733–1740 doi:10.1001/archinte.154.15.1733
Fabi A, Barduagni M, Lauro S et al (2003) Is delayed chemotherapy-induced emesis well managed in oncological clinical practice? An observational study. Support Care Cancer 11:156–161
Drug Utilization Review Team in Oncology (DURTO) (2003) Antiemetic prescription in Italian breast cancer patients submitted to adjuvant chemotherapy. Support Care Cancer 11:785–789 doi:10.1007/s00520-003-0478-0
Kaiser R (2005) Antiemetic guidelines: are they being used? Lancet Oncol 6:622–625 doi:10.1016/S1470-2045(05)70284-9
Oechsle K, Muller M, Hartmann J et al (2006) Aprepitant as salvage therapy in patients with chemotherapy-induced nausea and emesis refractory to prophylaxis with 5-HT3 antagonists and dexamethasone. Onkologie 29:557–561 doi:10.1159/000096689
Acknowledgement
This investigation was supported in part by a grant from Merck.
Author information
Authors and Affiliations
Corresponding author
Additional information
Brian Trainor is already deceased.
Rights and permissions
About this article
Cite this article
Hesketh, P.J., Younger, J., Sanz-Altamira, P. et al. Aprepitant as salvage antiemetic therapy in breast cancer patients receiving doxorubicin and cyclophosphamide. Support Care Cancer 17, 1065–1070 (2009). https://doi.org/10.1007/s00520-008-0545-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0545-7