Abstract
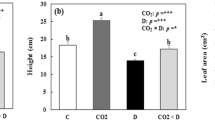
To test whether the impact of an enriched-CO2 environment on the growth and biomass allocation of first-season Quercus suber L. seedlings can modify the drought response under shade or sun conditions, seedlings were grown in pots at two CO2 concentrations × two watering regimes × two irradiances. Compared to CO2, light and water treatment had greater effects on all morphological traits measured (height, stem diameter, number of leaves, leaf area, biomass fractions). Cork oak showed particularly large increases in biomass in response to elevated CO2 under low-watered (W−) and high-illuminated conditions (L+). Allocation shifted from shoot to root under increasing irradiance (L+), but was not affected by CO2. Changes in allocation related to water limitation were only modest, and changed over time. Relative growth rate (RGR) and net assimilation rate (NAR) were significantly greatest in the L+/W+ treatment for both CO2 concentrations. Changes in RGR were mainly due to NAR. Growth responses to increased light, water or CO2 were strongest with light, medium with water availability and smallest for CO2, in terms of RGR. The rise in NAR for light and water treatments was counterbalanced by a decrease in SLA (specific leaf area) and LMF (leaf mass fraction). Results suggest that elevated CO2 caused cork oak seedlings to improve their performance in dry and high light environments to a greater extent than in well-irrigated and low light ones, thus ameliorating the effects of soil water stress and high light loads on growth.





Similar content being viewed by others
Abbreviations
- RGR:
-
Relative growth rate
- NAR:
-
Net assimilation rate
- LMF:
-
Leaf mass fraction
- RMF:
-
Root mass fraction
- SMF:
-
Stem mass fraction
- GRC:
-
Growth response coefficient
- SLA:
-
Specific leaf area
- S/R:
-
Shoot-to-root ratio
References
Abrams MA, Kloeppel BD, Kubiske ME (1992) Ecophysiological and morphological responses to shade and drought in two contrasting ecotypes of Prunus serotina. Tree Physiol 10:343–355
Ainsworth EA, Long SP (2005) Tansley Review: What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–357
Aranda I, Castro L, Pardos M, Gil L, Pardos JA (2005) Effects of the interaction between drought and shading on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. For Ecol Manag 210:117–129
Aranda I, Pardos M, Puértolas J, Jiménez MD, Pardos JA. 2006. Water use efficiency in cork oak (Quercus suber L.) is modified by the interaction of water and light availabilities. Tree Physiol (in press)
Arnone JA, Körner C (1995) Soil and biomass carbon pools in model communities of tropical plants under elevated CO2. Plant Cell Environ 14:869–875
Atkin OK, Schortemeyer M, McFarlane N, Evans JR (1998) Variation in the components of relative growth rate in ten Acacia species from contrasting environments. Plant Cell Environ 21:1007–1017
Atkin OK, Schortemeyer M, McFarlane N, Evans JR (1999) The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia 120:544–554
Biel C, Savé R, Habrouk A, Espelta JM, Retana J (2004) Effects of restricted watering and CO2 enrichment in the morphology and performance after transplanting of nursery-grown Pinus nigra seedlings. HortScience 39:535–540
Bormann FH, Likens GE (1994) Pattern and process in a forested ecosystem. Disturbance, development and the steady state based on the Hubbard Brook ecosystem study, 2nd edn. Springer-Verlag, New York, 253 pp
Brouwer R (1983) Functional equilibrium: sense or nonsense? Neth J Agric Sci 31:335–348
Catovsky S, Bazzaz FA (1999) Elevated CO2 influences the responses of two birch species to soil moisture: implications for forest community structure. Global Change Biol 5:507–518
Climent J, Aranda I, Alonso J, Pardos JA, Gil L (2006) Developmental constraints limit the response of Canary Island pine seedlings to combined shade and drought. For Ecol Manag (in press)
Cortes P, Espelta JM, Savé R, Biel C (2004) Effects of a nursery CO2 enriched atmosphere on the germination and seedlings morphology of two Mediterranean oaks with contrasting leaf habitat. New Forest 28:79–88
Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Drake BG, González-Meer MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Ann Rev Plant Physiol Plant Mol Biol 48:609–639
Gregory KM (1996) Are paleoclimate estimates biased by foliar physiognomic responses to increased atmospheric CO2? Palaeoecogr Palaeoclimat Palaeoecol 124:39–51
Hättenschwiler S, Körner C (2000) Tree seedling responses to in situ CO2-enrichment differ among species and depend on understorey light availability. Global Change Biol 6:213–226
Hättenschwiler S, Körner C (2003) Does elevated CO2 facilitate naturalization of the non-indigenous Prunus laurocerasus in Swiss temperate forests? Funct Ecol 17:778–785
Houghton JT, Ding Y, Griggs DJ, Noguer M, Van Der Linden PJ, Xiaosu D (eds) (2001) Climate change 2001: the scientific basis. Cambridge University Press. Earth, environment and atmospheric sciences, 944 p.
Hunt R (1990) Basic growth analysis. Plant growth analysis for beginners. Unwin Hyman, London, 112 p.
Hunt R, Cornelissen JHC (1997) Components of relative growth rate and their interrelation in 59 temperate plant species. New Phytol 135:395–417
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54(2):187–211
Idso SB, Kimball BA (1992) Effects of atmospheric CO2 enrichment on photosynthesis, respiration, and growth of sour orange trees. Plant Physiol 99:341–343
Idso SB, Kimball BA (1997) Effects of long-term atmospheric CO2 enrichment on the growth and fruit production or sour orange trees. Global Change Biol 3:89–96
Idso SB, Kimball BA, Hendrix DL (1993) Air-temperature modifies the size-enhancing effects at atmospheric CO2 enrichment on sour orange tree leaves. Environ Exp Bot 33:293–299
King JS, Thomas RB, Strain BR (1997) Morphology and tissue quality of seedling root systems of Pinus taeda and Pinus ponderosa as affected by varying CO2, temperature, and nitrogen. Plant Soil 195:107–119
Kitaya Y, Niu G, Kozai T, Ohashi M (1998) Photosynthetic photon flux, photoperiod and CO2 concentration affect growth and morphology of lettuce plug transplants. Hortscience 33:988–991
Lambers H, Cambridge ML, Konings H, Pons TL (eds) (1990) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic Publishing, The Hague, The Netherlands, 363 p
Lindström MJ, Bates DM (1990) Nonlinear mixed effects for repeated measures data. Biometrics 46:673–687
Llorens L, Peñuelas J, Estiarte M (2003) Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiol Plant 119:231–243
Löf M, Bolte A, Welander T (2005) Interacting effects of irradiance and water stress on dry weight and biomass partitioning in Fagus sylvatica seedlings. Scand J For Res 20:322–328
McKenna MF, Shipley B (1999) Interacting determinants of interspecific relative growth: empirical patterns and a theoretical explanation. Ecoscience 6:286–296
Montero G, Cañellas I (2003) Selvicultura de los alcornocales en España. Silva Lusit 11:1–19
Nagy M, Ogawa K, Hagihara A (2000) Interactive effect of CO2 enrichment and temperature on the photosynthesis of field-grown hinoki cypress (Chamaecyparis obtusa) branches. Trees Struct Funct 14:282–288
Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R (1999) Tree responses to rising CO2 in field experiments: implications for the future forest. Plant Cell Environ 22:683–714
Norby RJ, Wullschleger SD, Gunderson CA, Nietch CT (1995) Increased growth efficiency of Quercus alba trees in a CO2-enriched atmosphere. New Phytol 131:91–97
Norby RJ (1994) Issues and perspectives for investigating root responses to elevated atmospheric carbon dioxide. Plant Soil 165:9–20
Pardos M, Jiménez MD, Aranda I, Puértolas J, Pardos JA (2005) Water relations of cork oak (Quercus suber L.) seedlings in response to shading and moderate stress. Ann For Sci 62:377–384
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Poorter L (1999) Growth responses of 15 rain-forest species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410.
Quero JL, Marañón T, Villar R (2004) Tasas de fotosíntesis en plántulas de alcornoque y roble en distintos micrositios dentro del sotobosque. Almoraima Comunicaciones 31:101–110
Rozema J (1993) Plant responses to atmospheric carbon dioxide enrichment: interactions with soil and atmospheric conditions. In: Rozema J, Lambers H, van der Geijn SC, Cambridge ML (eds) CO2 and biosphere, vols 104/105. Vegetatio, pp 173–190
Sack L, Grubb PJ (2002) The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 131:175–185
Shipley B (2002) Trade-offs between net assimilation rate and specific leaf area in determining growth rate: relationship with daily irradiance. Funct Ecol 16:682–689
Stulen I, Den Hertog J (1993) Root growth and functioning under CO2 enrichment. Vegetatio 104–105:99–115
Tingey DT, Phillips DL, Johnson MG (2000) Elevated CO2 and conifer roots: effects on growth, life span and turnover. New Phytol 147:87–103
Usami T, Lee J, Oikawa T (2001) Interactive effects of increased temperature and CO2 on the growth of Quercus myrsinaefolia saplings. Plant Cell Environ 24:1007–1019
Valladares F, Pearcy R (1997) Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ 20:25–36
Valladares F, Pearcy R (2002) Drought can be more critical in the shade than in the sun: a field study of carbon gain and photo-inhibition in a Californian shrub during a dry El Niño year. Plant Cell Environ 25:749–759
Veenendaal EM, Swaine MD, Lecha RT, Walsch MF, Abebrese IK, Owusu-Afriyie K (1996) Responses of West African forest tree seedlings to irradiance and soil fertility. Funct Ecol 10:501–511
Wang JR, Hawkins CDB, Letchford T (1998) Relative growth rate and biomass allocation of paper birch (Betula papyrifera) populations under different soil moisture and nutrient regimes. Can J For Res 28:44–55
Walters MB, Reich PB (2000) Seed size, nitrogen supply, and growth rate affect tree seedling survival in deep shade. Ecology 81:1887–1901
Yin X (2002) Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Global Change Biol 8:631–642
Acknowledgements
We thank Rafael Calama for statistical advice. This study was financially supported by AGL-2720 project, Plan Nacional I+D+I.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Karnosky
Rights and permissions
About this article
Cite this article
Pardos, M., Puértolas, J., Aranda, I. et al. Can CO2 enrichment modify the effect of water and high light stress on biomass allocation and relative growth rate of cork oak seedlings?. Trees 20, 713–724 (2006). https://doi.org/10.1007/s00468-006-0086-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-006-0086-y