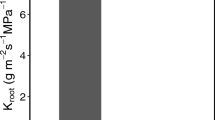Abstract
Leaf and bud demography and shoot growth were studied in 10 evergreen (ES) and 15 deciduous (DS) tree species occurring between 600 and 2200 m elevation in the central Himalayan mountains in India. Results were analyzed to help explain why ES prevail in the vegetation of this region, even though the number of ES is no greater than for DS. Although each species had its own pattern with regard to leaf and bud demography and seasonality of shoot extension and radial growth, it was possible to group the species on the basis of shoot growth phenology. In most species, leaves emerged during March-April, at the onset of warm and dry summer season. The ES recruit leaves in shoots more rapidly than the DS. Across all species, peak number of leaves per shoot (5.8–20.7), peak leaf area per shoot (116.2–1559.2 cm2), peak number of vegetative buds per shoot (1.9–14.5), bud survival per shoot (23–84%), shoot extension growth (6.4–40.8 cm) and shoot extension period (13–30 weeks) varied considerably. The peak leaf area per shoot (587.7 vs. 246.7 cm2) and shoot extension growth (19.3 vs. 11.2 cm) were significantly greater for DS than for ES, and these two functional groups of species were clearly separable with regard to shoot growth characteristics.
Results indicate that rapid recruitment of leaf crop in the shoots, longer leaf life-span, and access to ground water due to deep roots were some of the advantages, the ES had over the DS, that may have likely enable them to maintain growth for a longer period in this region of warm winters and longer winter day length as compared to temperate climates. In the shallow rooted DS, shoot growth seems to be much affected by a seasonal drought in winter and they are likely to be affected more in the event of failure of monsoon rains in this region.






Similar content being viewed by others
References
Abrams MD (1990) Adaptations and responses to drought in Quercus species of North America. Tree Physiol 7:227–238
Ahlgren CE (1957) Phenological observations of nineteen native tree species in northeastern Minnesota. Ecology 38:622–628
Arroyo M, Armesto JJ, Villagran C (1981) Plant phenological patterns in the high Andean Cordillera of Central Chile. J Ecol 69:205–223
Baker TR, Affum-Baffoe K, Burslem DFRP, Swaine MD (2002) Phenological differences in tree water use and the timing of tropical forest inventories: conclusions from patterns of dry season diameter change. For Ecol Mgmt 171:261–274
Bazzaz FA, Harper JL (1977) Demographic analysis of the growth of Linum usitatissimum. New Phytol 78:193–208
Bhadula SK, Joshi SC, Purohit AN (1995) Seasonal variation in photosynthetic characteristics of some mountain tree species from Garhwal Himalaya. Physiol Mol Biol Pl 1:151–160
Boojh R, Ramakrishnan PS (1982) Growth strategy of trees related to successional status II Leaf dynamics. For Ecol Mgmt 4:375–386
Borchert R (1994) Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75:1437-1449
Borchert R, Rivera G, Hagnauer W (2002) Modification of vegetative phenology in a tropical semi-deciduous forest by abnormal drought and rain. Biotropica 34:27–39
Chabot BF, Hicks AJ (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13:229–259
Champion HG, Seth SK (1968) A revised survey of the forest types of India. Govt. of India Publ. New Delhi
Chapin FS III, Tryon PR (1983) Habitat and leaf habit as determinants of growth, nutrient absorption, and nutrient use by Alaskan taiga forest species. Can J For Res 13:818–826
Critchfield WB (1960) Leaf dimorphism in Populus trichocarpa. Am J Bot 47:699–711
Dhaila S (1991) Phenology of deciduous and evergreen broadleaf species with particular reference to water stress. Ph.D. Thesis, Kumaun Univ. Nainital, India
Dhaila S, Singh SP, Negi GCS, Rawat YS (1995) Shoot growth phenology of co-existing evergreen and deciduous species in an oak forest. Ecol Res 10:151–159
Fife DN, Nambiar EKS (1982) Accumulation and retranslocation of mineral nutrients in developing needles in relation to seasonal growth of young radiate pine trees. Ann Bot 50:817–829
Frankie GW, Baker HG, Opler PA (1974) Comparative phonological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 62:881–919
Fraser DA (1956) Ecological studies of forest trees at Chalk River, Ontario, Canada. II. Ecological conditions and radial increment. Ecology 37:777–789
Gholz HL, Fitz FK, Waring RH (1976) Leaf area differences associated with old-growth forest communities in the western Oregon Cascades. Can J For Res 6:49–57
Gill A (1971) The formation, growth, and fate of buds of Fraxinus americana L. in central Massachusetts. Harvard Forest Paper 20. Harvard Univ. Petersham, Mass
Gill DS, Mahall BE (1986) Quantitative phenology and water relations of an evergreen and a deciduous chaparral shrub. Ecol Monogr 56:127–143
Givnish TJ (2002) Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fennica 63:703–743
Gray JT (1983) Nutrient use by evergreen and deciduous shrubs in southern California. I. Community nutrient cycling and nutrient-use efficiency. J Ecol 71:21–41
Halle F, Oldeman RAA, Tomlinson PB (1978) Tropical trees and forests. Springer-Verlag, Berlin
Hall JB, Swaine MD (1981) Distribution of ecology of vascular plants in a tropical rain forest. Dr. W. Junk, The Hague
Hanninen H (1995) Effects of climatic change on trees from cool and temperate regions: an ecophysiological approach to modeling of bud burst phenology. Can J Bot 73:183–199
Hilty SL (1980) Flowering and fruiting periodicity in a pre-montane rain forest in Pacific Columbia. Biotropica 13:292–306
Hinckley TM, Lassoie JP, Running SW (1978) Temporal and spatial variations in the water status of forest trees. For Sci Monogr 20. Soc Amer For Washington, D.C. USA
Khanna RK (1986) Standing state of nutrients in different forest ecosystems of Kumaun Himalaya. Ph D Thesis, Kumaun Univ. Naini Tal, India
Kikuzawa K (1978) Emergence, defoliation and longevity of Alder (Alnus hirsuta Turcz.) leaves in a deciduous hardwood forest stand. Jap J Ecol 28:299–306
Kikuzawa K (1995) The basis for variation in leaf longevity of plants. Vegetatio 121:89–100
Koppen W (1931) Grundriss der Klimakunda (2nd Edition)
Kozlowski TT (1971) Growth and development of trees. Vol I. Academic Press, New York.
Kozlowski TT (1972) Shrinking and swelling of plant tissues. In: Kozlowski TT (ed) Water deficits and plant growth, Academic Press, New York, pp 1–64
Kozlowski TT, Winget CH, Torrie JH (1962) Daily radial growth of oak in relation to maximum and minimum temperature. Bot Gaz 124:9–17
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Kudo G (1995) Altitudinal effects on leaf traits and shoot growth of Betula platyphylla var. japonica. Can J For Res 25:1881–1885
Lanner RM (1976) Patterns of shoot development in Pinus and their relationship to growth potential. In: Canell MGR, Last FT (eds) Tree physiology and yield improvement. Academic Press, New York, pp 223–243
Lawton JRS, Akpan EEJ (1968) Periodicity in Plumeria. Nature 218:384–386
Lechowicz MJ (1984) Why do temperate deciduous trees leaf out at different times? Adaptation and ecology of forest communities. Amer Nat 124:821–842
Longman KA, Jenik J (1974) Tropical forest and its environment. Longman, London and New York
Maillette L (1982) Structural dynamics of silver birch. I. The fate of buds. J Appl Ecol 19:203–218
Marques MCM, Roper JJ, Salvalaggio APB (2004) Phenological patterns among plant life-forms in a sub-tropical forest in southern Brazil. Plant Ecol 173:203–213
Maruyama K (1978) Shoot elongation characteristics and phenological behaviour of forest trees in natural beech forest. Bull Niigata Univ For 12:19–41
McGraw JB, Antonovics J (1983) Experimental ecology of Dryas octopetula ecotypes. I. Ecotypic differentiation and life-cycle stages of selection. J Ecol 71:879–897
Mooney HA, Dunn EL (1970) Convergent evolution of mediterranean-climate evergreen sclerophyll shurbs. Evolution (Lawerence, Kans) 24:292–303
Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Ann Rev Ecol Syst 17:67–88
Mulkey SS, Smith AP, Wright SJ, Machado JL, Dudley R (1992) Contrasting leaf phenotypes control seasonal variation in water loss in a tropical forest shrub. Proc Natl Acad Sci USA 89:9084–9088
Myers BA, Duff GA, Eamus D, Fordyce IR, O'Grady A, Williams RJ (1997) Seasonal variation in water relations of trees of differing leaf phenology in a wet – dry tropical savanna near Darwin, Northern Australia. Aus J Bot 45:225–240
Negi GCS (1989) Phenology and nutrient dynamics of tree leaves in Kumaun Himalayan forests. Ph D Thesis. Kumaun Univ. Nainital, India
Negi GCS, Singh SP (1992) Leaf growth pattern in evergreen and deciduous species of the Central Himalaya, India. Int J Biometerol 36:233–242
Negi GCS, Singh SP (1993) Leaf nitrogen dynamics with particular reference to retranslocation in evergreen and deciduous tree species of Kumaun Himalaya. Can J For Res 23:349–357
Nelson JA, Barnes PW, Archer S (2002) Leaf demography and growth responses to altered resource availability in woody plants of contrasting leaf habit in a subtropical savanna. Plant Ecol 160:193–205
Nilsen ET, Sharifi MR, Rundel RW (1987) Leaf dynamics in an evergreen and a deciduous species with even-aged leaf cohorts from different environments. Amer Midland Naturalist 118:46–55
Njoku E (1963) Seasonal periodicity in the growth and development of some forest trees in Nigeria. I. Observations on mature trees. J Ecol 51:617–624
Opler PA, Frankie GW, Baker HG (1976) Rainfall as a factor in the release, timing and synchronization of anthesis by tropical trees and shrubs. J Biogeogr 3:231–236
Pook EW (1984) Canopy dynamics of Eucalyptus maculata Hook. I. Distribution and dynamics of leaf population. Aust J Bot 32:387–403
Poudyal K, Jha PK, Zobel DB, Thapa CB (2004). Patterns of leaf conductance and water potential of five Himalayan tree species. Tree Physiol 24:689–699
Ralhan PK, Khanna RK, Singh SP, Singh JS (1985) Phenological characteristics of the tree layer of Kumaun Himalayan forests. Vegetatio 60:91–101
Rawal RS, Bankoti NS, Samant SS, Pangtey YPS (1991) Phenology of tree layer species from the timber line around Kumaun in Central Himalaya, India. Vegetatio 93:109–118
Reich PB, Teskey RO, Johnson PS, Manbeck LJ, Hinckley TM (1978) Periodic root and shoot growth in oak. Proc North Amer For Biol Workshop 5:415–416
Reich PB, Borchert R (1982) Phenology and ecophysiology of the tropical tree, Tabebuia neochrysantha (Bignoniaceae). Ecology 63:294–299
Reich PB, Borchert R (1984) Water stress and tree phenology in a tropical dry forest in the lowlands of Costa Rica. J Ecol 72:61–74
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Nat Acad Sci USA 94:13730–13734
Rivera G, Elliott S, Caldas LS, Nicolossi G, Coradin VT, Borchert R (2002) Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Tree 16:445–456
Rook DA, Corson MJ (1978) Temperature and irradiance and the total daily photosynthetic production of a Pinus radiata tree. Oecologia 36:371–383
Saeki T, Nomoto N (1958) On the seasonal change of photosynthetic activity of some deciduous and evergreen broadleaf trees. Bot Mag (Tokyo) 71:235–241
Schwartz MD (1999) Advancing to full bloom: planning phenological research for the twenty-first century. Int J Biometerorol 42:113–118
Shaver GR (1981) Mineral nutrition and leaf longevity in an evergreen shrub, Ledum palustre ssp. Decumbens. Oecologia (Berl) 49:362–365
Shukla RP, Ramakrishnan PS (1982) Phenology of trees in a sub-tropical humid forest in north-eastern India. Vegetatio 49:103–109
Singh JS, Singh SP (1987) Forest vegetation of the Himalaya. Bot Rev 52:80–192
Singh SP, Rawat YS, Rana BS, Negi GCS (1990) Effects of unusually large seed crop on litterfall and nitrogen retranslocation in Himalayan oaks. For Ecol Mgmt 32:79–86
Singh JS, Singh SP (1992) Forests of Himalaya: structure, functioning and impact of man. Gyanodaya Prakashan, Nainital, India
Singh SP, Adhikari BS, Zobel DB (1994) Biomass, productivity, leaf longevity and forest structure in the central Himalaya. Ecol Monogr 64:401–421
Singh SP, Tewari A, Singh SK, Pathak GC (2000) Significance of phenologically asynchronous populations of the central Himalayan oaks in drought adaptation. Curr Sci 79:353–357.
Snedecor GW, Cochran WG (1967) Statistical methods-Oxford and IBH Publishing. Co Pvt Ltd New Delhi, India
Sobrado MA (1986) Aspects of tissue water relations and seasonal changes of leaf water potential components of evergreen and deciduous species coexisting in tropical dry forests. Oecologia 68:413–416
Tewari A (1998) Timing of drought: Effects of water relation of certain major forest types of lower and middle central Himalaya. Ph D Thesis, Kumaun University, Naini Tal
Tomlinson PB (1978) Branching and axis differentiation in tropical trees. In: Tomlinson PB, Zimmermann MH (eds) Tropical trees as living systems. Cambridge Univ Press, Cambridge, pp 187–208
Troup RS (1921) The silviculture of Indian trees. Vol. I–III. Clarendon Press, Oxford
Vitousek PM, Reiners WA (1975) Ecosystem succession and nutrient relation: a hypothesis. Bio Science 25:376–384.
Whitmore TC (1979) Tropical rain forests of the far east. Clarendon Press, Oxford
Williams-Linera G (2000) Leaf demography and leaf traits of temperate-deciduous and tropical evergreen-broadleaf trees in a Mexican montane cloud forest. Plant Ecol 149:233–244
Winget CH, Kozlowski TT (1965) Seasonal basal growth area as an expression of competition in northern hardwoods. Ecology 46:786–793
Wright SJ, Van Schaik CP (1994) Light and the phenology of tropical trees. Amer Nat 143:192–199
Wolfe JA (1979) Temperature parameters of humid and mesic forests of eastern Asia and relation to forests of other regions of the Northern hemisphere and Australasia. US Geol Survey Prof Paper 1106
Yavitt JB, Wright SJ, Wieder RK (2004) Seasonal drought and dry-season irrigation influence leaf-litter nutrients and soil enzymes in a moist, lowland forest in Panama. Aust Ecol 29:177–188
Zimmermann MH, Brown CL (1971) Trees: structure and function. Springer-Verlag. Berlin Heidelberg, New York
Zobel DB (1974) Local variation in intergrading Abies grandis-Abies concolor populations in the Central Oregon Cascades. II. Stomatal reaction to moisture stress. Bot Gaz 135:200–210
Zobel DB, Singh SP (1995) Tree water relations along the vegetational gradient in the Himalayas. Curr Sci 68:742–745
Zobel DB, Singh SP (1997) Himalayan forests and ecological generalizations. BioScience 47:735–745
Zobel DB, Garkoti SC, Singh SP, Tewari A, Negi CMS (2001) Patterns of water potential among forest types of the central Himalaya. Curr Sci 80:774–779
Acknowledgements
This study was carried out under the supervision of Prof. S.P. Singh, FNA, Head, Botany Department, Kumaun University, Naini Tal, India. Author is thankful to Prof. D.B. Zobel of Oregon State University, USA for reviewing a draft copy of this paper, and to the Director, G.B. Pant Institute of Himalayan Environment and Development, Kosi-Almora for providing facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. von Arnold
Rights and permissions
About this article
Cite this article
Negi, G.C.S. Leaf and bud demography and shoot growth in evergreen and deciduous trees of central Himalaya, India. Trees 20, 416–429 (2006). https://doi.org/10.1007/s00468-006-0056-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-006-0056-4