Abstract
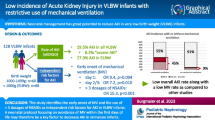
The aim of this study was to determine the incidence, risk factors, and outcome of acute kidney injury (AKI) in extremely low birth weight (ELBW) infants. In a case–control study, medical records of all ELBW infants who were admitted to our Neonatal Intensive Care Unit (NICU) between 1 January 2000 and 31 January 2008 were reviewed. During the study period, 12.5% (59/472) of all ELBW infants developed AKI. Forty-six infants with available medical records were matched to 46 controls. The mean gestational age and birth weight of infants with AKI and their controls were 24.7 ± 1.8 vs. 24.9 ± 1.9 weeks (p = 0.61) and 614 ± 128 vs. 616 ± 127 g (p = 0.93), respectively. Infants with AKI had a higher mean airway pressure, a lower mean arterial blood pressure, and higher exposure to cefotaxime than their controls. Infants with AKI also had an increased mortality in comparison to their controls [33/46 (70%) vs. 10/46 (22%), respectively; p < 0.0001), and oliguric patients had a higher mortality than nonoliguric patients [31/38 (81%) vs. 2/8 (25%), respectively, p = 0.003]. Based on our results, we conclude that a high mean airway pressure, low blood pressure, and the use of cefotaxime are associated with renal failure in ELBW infants. AKI in ELBW infants is also associated with an increased mortality, especially in the presence of oliguria.
Similar content being viewed by others
Introduction
Improvements in the survival rates of very low birth weight (VLBW) and extremely low birth weight (ELBW) infants have paralleled improvements in perinatal and neonatal care and technology over the last two decades [1, 2]. However, there have been no significant increases in survival without neonatal and long-term morbidities [2, 3].
Acute renal failure or acute kidney injury (AKI) is defined as a rapid deterioration of normal renal function, resulting in the retention of nitrogenous wastes and other biochemical derangements. AKI affects 3.4–24% of all infants who are admitted to the neonatal intensive care units (NICU) [4–6]. Several potential risk factors for the development of AKI in neonates have been identified [4, 7, 8]. Pre-renal mechanisms account for 85% of all AKI in this age group; thus, the functional integrity of the kidney is usually preserved, and renal failure is reversible with restoration of the underlying hemodynamic abnormality [4, 9, 10]. Intrinsic renal and post-renal failures are less frequent in neonates, accounting for 11 and 3%, respectively, of all cases [4]. In comparison to term infants, premature infants are at higher risk for AKI because of prenatal fetal distress and exposure to multiple risk factors such as infections, intrauterine growth retardation, placental insufficiency, and maternal medications. In addition, the postnatal course of premature infants is often complicated by the need for cardio–respiratory support, hypotension, and hypoxia [4, 10–12]. Prematurity by itself is an independent risk factor for AKI as the result of an incomplete nephrogenesis and low number of nephrons [4, 10, 11, 13], immature vasoregulation with high renal vascular resistance, high plasma rennin activity, low glomerular filtration rate (GFR), decreased inter-cortical perfusion, and decreased sodium reabsorption by the proximal tubules with a consequent increased susceptibility to hypoperfusion [12, 14–16].
Although the risk of developing AKI in ELBW infants is elevated, it has not been well studied. Therefore, in this study, our main objective was to determine the prenatal and postnatal risk factors associated with AKI in ELBW infants; our secondary objectives were to determine the incidence and outcomes of AKI in this age group.
Methods
This was a retrospective, case–control study in which all ELBW infants who were admitted to our Neonatal Intensive Care Unit (NICU) and developed AKI during their NICU stay between 1 January 2000 and 31 January 2008 were identified from our electronic medical database. Acute renal failure or AKI was defined as oliguria of <1 ml/kg/h of urine output that developed 24 h after birth and persisted for at least 24 h and/or a raising of the serum creatinine level >1.5 mg/dl 72 h after birth in the presence of normal maternal creatinine levels [17]. The day patients developed renal failure was defined as the index date. Since AKI in ELBW infants is a rare condition, and to avoid noncontemporary controls, each patient with AKI (case) was matched to a control patient (one control) who was born during the same period and whose weight was ±10% of the weight of the case patient and whose gestational age (GA) was that ± 1 week of the case patient. Our exclusion criteria consisted of all infants who had major congenital abnormalities and infants who were <23 weeks GA.
All medical records were reviewed for infants’ demographics [including GA, birth weight, small for gestational age (SGA), gender, race, mode of delivery, Apgar scores at 1 and 5 min, severity of illness as the Score for Neonatal Acute Physiology (SNAP) and SNAP Perinatal Extension (SNAPPE)], possible prenatal risk factors for AKI (including maternal age, pre-eclampsia, hypertension, maternal diabetes, maternal urinary tract infection, chorio-amnionitis, and maternal kidney dysfunction), and prenatal drug exposure [including prenatal steroid, beta-agonist, beta-blockers, calcium channel blocker, NSAIDs (nonsteroidal anti-inflammatory drugs), magnesium sulfate, and antibiotics ]. The SNAPPE score is a 9-item neonatal illness severity and mortality risk score. It is calculated from data collected on the day of admission to the NICU, and points are given for physiological items, birth weight, low Apgar score, and SGA [18].
Medical records were also reviewed for the presence of intra-ventricular hemorrhage (IVH), pneumothorax, days of mechanical ventilation, use of postnatal steroids, placement of umbilical arterial and venous lines, patent ductus arteriosus (PDA), blood culture-positive sepsis, necrotizing enterocolitis (NEC), renal ultrasound, and use of medications, such as benzodiazepines (midazolam, lorazepam), narcotics (morphine, fentanyl), dopamine, dobutamine, NSAIDs (indomethacin, ibuprofen), diuretics (furosemide, thiazides), xanthenes (theophylline, caffeine), antibiotics (gentamicin, vancomycin, ampicillin, cefotaxime), and antifungal (Amphotericin B). Total daily fluid intake, urine output, blood pressure, mean airway pressure, serum blood urea nitrogen (BUN), and serum creatinine were collected on the index date for the AKI group and the corresponding date for the control group.
The periods of exposure to the different risk factors in the control group were matched to the periods that preceded the development of AKI in the case group (for example, if a case infant developed AKI on day of life 10, the matched control risk factor exposures corresponded to the first 10 days of life). All variables (or risk factors for AKI) were selected in patients with AKI (cases) and controls if they occurred during the period that preceded the development of AKI in case patients or during the corresponding period in controls.
Patients’ outcomes, including mortality rate, and data on BUN, serum creatinine, blood pressure, and weight at discharge from the NICU among survivors were collected.
The study was approved by the Institutional Review Board at MetroHealth Medical Center.
Statistical analysis
A bivariate analysis was performed to identify differences between the case and the control groups. Student’s t test was used for parametric continuous variables, and the Mann–Whitney U test was used for nonparametric continuous variables. Chi-square tests and Fisher exact tests were used for categorical variables, as appropriate. All quantitative data were expressed as the mean ± standard deviation (SD), mean ± standard error of the mean (SEM), or median with inter-quartile range. A p ≤ 0.05 was considered to be statistically significant. A block entry of variables known to affect renal function and found to have a statistically significant difference between the two groups in the bivariate analysis were entered into a logistic regression model with AKI as the dependent variable. Continuous data of independent variables were dichotomized by choosing the 75% percentile of their mean as cutoff values. IBM SPSS Statistics ver. 19 (SPSS, Chicago, IL) was used for the statistical analysis of the data.
Results
During the study period 472 ELBW infants were admitted to the NICU, and 59 infants developed AKI, attaining an incidence of 12.5% (59/472). However only 46 patients had their medical record available for review and who could be matched to 46 control patients. All 46 patients with AKI met our inclusion criteria. As shown in Table 1, there were no significant differences in the demographics between the patients in the two groups. There were also no significant differences in prenatal risk factors and prenatal drug exposure (Table 2) between the two groups. However, in the postnatal period, in comparison to the control group, infants who developed AKI were exposed to a higher mean airway pressure within 24 h of the development of AKI, as shown in Table 3. Also in the postnatal period, in comparison to the control group, infants who developed AKI had a significantly higher exposure to cefotaxime, benzodiazepines, diuretics, and dopamine/dobutamine prior to the development of AKI in the case group and the corresponding period in the control group, as shown in Table 3.
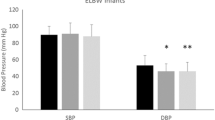
Within 24 h of the development of AKI in the case group and the corresponding period in the control group, there were significant differences in systolic, diastolic, and mean arterial blood pressures between the two groups, as shown in Fig. 1. The mean lowest mean arterial blood pressure recorded on the day of AKI or in the corresponding period in the control group was lower in the AKI than the control group (25.9 ± 9.9 vs. 35.5 ± 13.7 mm Hg, respectively; p < 0.001). There were no differences in available daily blood pressures between the two groups during the week that preceded the index date.
Average systolic (SBP), diastolic (DSP) and mean arterial (MAP) blood pressures in extremely low birth weight (ELBW) Infants within 24 h prior to the development of acute renal failure (ARF) or in the corresponding period in the control group (at the same postnatal age). Asterisk indicates that difference between groups is significant at p = 0.03
To adjust for possible confounders, we developed a binary logistic regression model with AKI as the dependent variable, and benzodiazepine, cefotaxime, diuretics, mean airway pressure (>10 cm H2O), mean arterial blood pressure (<21 mm Hg), and dopamine/dobutamine as independent variables. A mean airway pressure of >10 cm H2O was chosen as a variable since 10 cm H2O corresponds to the 75% percentile of the mean airway pressures of the studied population, and a mean arterial blood pressure of 21 mm Hg was chosen, since 21 mm Hg corresponds to the 25% percentile of the mean arterial blood pressures of the studied population. After adjustment for variables that were significant in a bivariate analysis, only exposure to cefotaxime, high mean airway pressure (>10 cm H2O) and low mean arterial blood pressure (<21 mm Hg) remained significant risk factors for the development of AKI in ELBW, as shown in Table 4. When an exploratory backward stepwise likelihood ratio analysis was conducted, with AKI as the dependent variable and phenobarbital, mean airway pressure, NEC, sepsis, cefotaxime, narcotics, inotropes/pressors, diuretics, benzodiazepines, and lowest mean arterial blood pressure as independent variables, the results were consistent with the block entry model. In the final step of the model, the lowest mean arterial blood pressure, mean airway pressure, and cefotaxime were the only independent variables that had a significant p value.
Renal ultrasound and Doppler flow studies were performed in 20/46 (43%) of the patients with AKI. The major ultrasound finding was renal medullary hyperechogenicity, which was found in 6//20 patients (30%). One patient had bilateral small kidneys, one patient had a mild unilateral pelviectasis, and one patient had an abdominal aortic thrombus without occlusion of the renal arteries.
None of the patients who had AKI developed hypertension. The total fluid intake within the 24-h period prior to the development of AKI or in the corresponding period in the control group was significantly higher in patients who developed AKI than in their controls (156 ± 68 vs. 131 ± 45 ml/kg/day; p = 0.042).
AKI (according to urinary output and/or serum creatinine levels) developed at 8.5 (2–16.5) days of life (median and interquartile range) and lasted for 5 (2–11.5) days (median and interquartile range). In patients with AKI, 38/46 (82.6%) were oliguric and 8/46 (17%) were nonoliguric and had a serum creatinine level >1.5 mg/dl. Overall, patients with AKI had a median serum creatinine and BUN level of 1.8 (1.5–2.35) mg/dl and 33 (22–45) mg/dl, respectively, on the day of their diagnosis. The duration of AKI was calculated as the number of days from the onset of AKI until death or improvement of the urinary output (>1 ml/kg/h) and serum creatinine level (<1.5 mg/dl). Among patients who had AKI and died, 29/33 had AKI at the time of death, and 4/33 had a resolution of their AKI at the time of their death.
Patients with AKI had a higher mortality than their controls [33/46 (70%) vs. 10/46 (22%), respectively; p < 0.001], and among AKI patients mortality was higher in oliguric versus nonoliguric patients [31/38 (81%) vs. 2/8 (25%), respectively, p = 0.003].
Among survivors, there were no significant differences in BUN, creatinine, blood pressures, or weight between AKI patients and their controls at discharge from the NICU, as shown in Table 5.
Discussion
The results of our study reveal that AKI is common in ELBW infants and that hypotension, elevated mean airway pressure, and the use of cephalosporin are associated with the development of AKI in ELBW infants. We have also shown that ELBW infants with AKI have a high mortality, especially if their AKI is associated with oliguria. To the best of our knowledge, our case–control study is the largest reported study on AKI in ELBW infants.
There is no universally accepted definition for neonatal AKI. Serum creatinine levels and reduced urine output (oliguria <1 ml/kg/h) are the most commonly used markers to identify acute renal failure or AKI [7]. We and others have previously shown that serum creatinine in ELBW infants usually rises in the immediate neonatal period due to inherited maternal creatinine load, reaches a peak on the second day, and then decline to normal levels over the next 1–2 weeks [19, 20]. However, a raising or persistent high level of serum creatinine >1.5 mg/dl after 48–72 h of life has been used as a definition for AKI [7, 17, 21–23].
The reported incidence of AKI in newborn infants admitted to NICUs has been reported to vary between 3.4 and 24%, which is consistent with our findings [4–6]. In a study of 359 premature infants born at <37 weeks of GA, Csaicsich et al. found that the incidence of AKI was 4.5% among all premature infants and 6.4% among ELBW infants (n = 156) [6]. In our study, the incidence of AKI was 12.5% among our 472 ELBW infants. One possible explanation for the difference between the incidence of AKI in our study and that of Csaicsich et al. is that our case group consisted exclusively of ELBW infants who were relatively smaller; for example, the average weight of ELBW infants studied by Csaicsich et al. was 725 g (n = 10), whereas the mean weight of our patients was 615 g (n = 46).
In newborn infants, AKI is predominantly associated with oliguria. The reported incidence of AKI with oliguria has varied between 46 and 93%, which is consistent with our findings [5, 15, 16, 22]. In our study, 82.6% of our patients had AKI associated with oliguria, and a small proportion had a nonoliguric AKI. The incidence of nonoliguric AKI has been reported to be higher in full term infants. Approximately one-third of all acute renal failures in full term infants are nonoliguric and in asphyxiated infants in particular, two thirds of all acute renal failures are nonoliguric [5, 9, 24, 25].
Prenatal use of nephrotoxic medications has been associated with AKI in premature infants. For example, studies and case reports have shown that prenatal exposure to NSAIDs is associated with nephrotoxicity and AKI in newborn infants [10, 23, 26, 27]. Cataldi et al. reported an increase in prenatal use of nephrotoxic medications, mainly antibiotics and NSAIDs, in preterm infants with AKI [10]. In our study, we did not find prenatal exposure to different nephrotoxic medications, including NSAIDs, to be associated with AKI in our ELBW patients. Our findings may differ from those of others because of differences in medical management and practices. For instance, only 6% of our patients’ mothers were exposed to NSAIDs versus 37% in Cataldi et al.’s study [10].
Low Apgar scores of <7 at 5 min of life have been reported to be a risk factor for AKI in GA infants ≤36 weeks and ≥34 weeks. [10, 23, 28]. In a study of infants ≥ 34 weeks GA, Aggarwal et al. reported an increase in the incidence of AKI in infants who were asphyxiated with a low 5-min Apgar scores of <6 in comparison to their controls (56 vs. 4%, respectively)[28]. In our study, we did not find a significant difference in Apgar scores between our cases and controls, most probably because we had a small number of infants who had low Apgar scores at 5 min (less than 25% of our patients had an Apgar score ≤5, as shown in the interquartile range of the median Apgar scores at 5 min of life in our patients). In a large retrospective cohort study, the mean 5-min Apgar score reported in ELBW infants was 6.6 ± 2.1 [29], which is comparable to that of our patients.
A clinically significant PDA can be responsible for AKI, either as a result of a large left to right shunting and aortic run-off causing renal hypo-perfusion or as a result of an attempted medical closure with NSAIDs [10, 30]. In our series, the incidence of PDA was similar between the groups. In our patients, medical closure was attempted in a small number of patients, and surgical ligation was performed in the majority of our patients. It is possible that our practice of early surgical ligation of PDAs in ELBW infants during the study period contributed to our lower incidence of PDA-related renal failure. Alexander et al. reported similar findings: i.e., surgically treated PDA was associated with less renal dysfunction than indomethacin-treated PDA in ELBW infants [31].
In preterm infants, 50% of all acute renal failures have been attributed to postnatal drug exposure [7]. Cataldi et al. demonstrated that infants with AKI were subjected to long-term exposure to antibiotics, NSAIDs, and diuretics [10]. The use of several drugs has been associated with AKI; for example, the use of angiotensin-converting enzyme inhibitors has been associated with AKI in newborn infants [32], and the use of midazolam has been associated with hypotension and oliguria [33]. In our study, the bivariate analysis revealed that infants with AKI had a higher postnatal exposure to cefotaxime, benzodiazepines, diuretics and dopamine/dobutamine prior to the development of AKI. However, following adjustment to statistically significant and relevant confounders in a logistic regression model, cephalosporin remained the only drug that was associated with AKI.
It has been speculated that loop diuretics via prostaglandin-induced vasodilatation may improve vasomotor nephropathy-related AKI; however, the efficacy of this intervention has not been confirmed in adult studies [34]. It has also been suggested that patients who respond to diuretics might have less severe AKI [34]. In our study, and following adjustment to possible confounders, the use of diuretics was not associated with AKI in our patients.
The higher exposure to cephalosporin prior to the development of AKI seen in our study is consistent with previous reports [10]. The nephrotoxicity of cephalosporin depends on the intra-cortical concentration of the drug, which in turn depends on tubular transport mechanisms. Therefore, any alteration of tubular transportation can potentially lead to nephrotoxic drug levels [35]. Most cephalosporins are safe for use in newborn infants; however, at extremely high levels, third-generation cephalosporins, which are the most common drugs used to treat newborn infants, may cause renal damage [35]. Unfortunately, drug levels of cephalosporins are not routinely monitored at the bedside, and the potential to reach high drug concentrations in the setting of vasomotor nephropathy is possible and might have contributed to worsening renal failure in our infants, especially given the fact that our patients with AKI had a lower mean arterial blood pressure than their controls.
The vasomotor nephropathy-related prerenal failure seen in neonates with respiratory disorders is often induced/aggravated by positive airway pressure ventilation [4, 7, 9, 10]. In our study, infants with AKI had a higher mean airway pressure than their controls, suggesting that infants with AKI had more severe respiratory distress symptoms, which is consistent with the findings of previous studies [4, 10]. Higher mean airway pressures in our AKI patients might have impaired the systemic venous return, decreased cardiac output, and decreased systemic blood pressure, leading to a decrease in renal perfusion.
In our study, infants with AKI had significantly lower arterial blood pressures and higher intravenous fluid intakes (within 24 h of the development of their renal failure) than their controls, suggesting that our AKI patients remained hemodynamically unstable in the period that preceded their renal failure despite fluid resuscitation. This observation emphasizes the importance of pre-renal kidney failure as the most common cause of AKI in ELBW infants [4, 9, 10]. However, since daily infants’ weights were not available to review, it would be difficult to determine if infants with AKI had adequate fluid resuscitation or not and if their need for a higher mean airway pressure was not a response to treat pulmonary edema and fluid overload.
To adjust for possible confounders, we developed a binary logistic regression model to study potential risk factors associated with AKI in premature infants, similar to the approach adopted by Cataldi et al. in their study [10]. In their multivariate logistic regression analysis, Cataldi et al. found that a low Apgar score, a medullary hyperechogenicity, ampicillin, ceftazidime, and ibuprofen were risk factors; in contrast, we found that high mean airway pressure, low blood pressure, and cefotaxime were associated with AKI in premature infants. Our results may differ from those of Cataldi et al. for at least two reasons. Cataldi et al. studied a larger and less premature population, and they used a different definition for acute renal failure [10]. In their study of 71 premature infants (<38 weeks GA) with acute renal failure, the average GA and birth weight were 27.8 weeks and 1115.9 g, respectively; in comparison, the average GA and birth weight of our patients were 24.7 weeks and 614.6 g, respectively. Therefore, our study completes the aforementioned study by analyzing only ELBW premature infants who might differ from older premature infants. The definition of AKI in our study was also different. Cataldi et al. defined AKI in infants <33 weeks GA as a serum creatinine level >114.92 micromol/l (or 1.3 mg/dl) with and without oliguria after 60 h of life; in comparison, our definition of AKI was an oliguria of <1 ml/kg/h of urine output developing after 24 h of birth or an increasing serum creatinine level >1.5 mg/dl after 72 h of birth [10]. Few of our cases met the criteria of AKI based on oliguria alone and would have been missed under the serum creatinine criteria only.
Our renal ultrasound findings of infants with AKI are consistent with previous findings and reports [10]. In our study, all patients who had renal imaging studies available for review had normal renal anatomy, with the exception of one patient who had small kidneys and one patient who had mild pelviesctasis. Our predominant renal ultrasound finding was renal medullary hyperechogenicity, which is usually associated with a medical renal disease or an index of nonspecific renal stress, a finding that is consistent with previous reports [10]. Aortic thrombosis-related AKI has been reported as a complication of umbilical arterial lines [10, 36–38]. The number of umbilical arterial lines was similar between the groups in our study. However, we had one patient with AKI who had an abdominal aortic clot, although the clot did not extend to the level of the renal arteries. Overall, our patients’ renal ultrasound findings are most probably secondary to the AKI rather than being an associated risk factor.
Most acute renal failures in neonates are secondary, transient, and reversible with the resolution of the underlying conditions, with the exception of a few cases of congenital or primary renal conditions [9, 10, 39–41]. A high mortality rate has been reported in neonate with AKI (range 25– 80%), which is consistent with our findings (our mortality rate was 70% in patients with AKI) [8, 15, 16, 23].
In our study, at the time of discharge from the NICU, there were no differences in serum creatinine, serum BUN, or blood pressures in survivors of AKI and their controls. However, long-term follow-up is necessary since ELBW infants, even without evidence of AKI in the neonatal period, are subject to a decrease in their GFR and tubular phosphate transportation, probably as a consequence of an impaired postnatal nephrogenesis [13]. Abitbol et al. conducted a long-term follow-up study of 20 ELBW infants with a history of AKI in the neonatal period and found that proteinuria (urine protein/creatinine >0.6 mg/dl), elevated serum creatinine (serum creatinine >0.6 mg/dl at 1 year of age), and tendency to obesity (with body mass index >85th percentile) are prominent risk factors for long-term progressive renal disease. Based on these findings, the authors emphasized the need for long-term follow-up of renal function in this highly vulnerable population [42].
Our study has multiple limitations. It is a retrospective, small-sized, single-center study that reviewed the outcome of ELBW infants over an 8-year time span. The management of premature infants has evolved over the years, and it is possible that the management of our patients has changed during the study period. However, we attempted to counteract this potential problem by designing our study as a case–control study with contemporary controls. An attempt to identify perfect matches to our cases may also be a limitation to our study with the potential of an over-matching bias. Given the retrospective design of our analysis, we can only describe associations between variables and can only state that our risk factors are only associated risk factors with AKI in ELBW—only prospective studies have the potential to address causality. For example, to be able to confirm that cephalosporin is a risk factor, a prospective controlled study is needed. The use of cephalosporin by the treating physicians could have been related to factors that are undetected by our analysis. Since the majority of ELBW infants require frequent blood tests for the management of their fluid and electrolytes, we relied on our medical records to identify infants with AKI. However, one of the limitations of our retrospective review is that potential cases of AKI could have been missed since we did not have a universal serum creatinine screening of all infants or serial renal studies (such as fractional excretion of sodium and urea), and since there is no universal definition for AKI in ELBW infants. Another limitation of our study is related to our reliance on serum creatinine as one of the criteria for the diagnosis of AKI. However, serum creatinine may be a late marker of renal failure. In the future, relying on urinary biomarkers may be more helpful in identifying renal failures prior to the rise in serum creatinine.
Although we did not find a difference in outcome at discharge between patients who developed AKI and their controls, our study was not designed or powered to study the outcome of AKI in ELBW infants. Future prospective, long-term, multi-institutional studies are needed to determine the long-term outcome of AKI in ELBW.
Conclusion
To the best of our knowledge our case–control study is the largest reported study on AKI in ELBW infants. In our study, we have shown that AKI is common in ELBW infants and that hypotension, elevated mean airway pressure, and the use of cephalosporin are associated with the development of AKI in ELBW infants. Early interventions, such as fluid resuscitation, to prevent hypotension and avoid high mean airway pressures should be considered in the management of ELBW infants at risk for the development of AKI.
References
Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK (2001) Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 107:E1
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK (2007) Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196:147.e1–147.e8
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID 3rd, Watterberg KL, Saha S, Das A, Higgins RD (2010) Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126:443–456
Hentschel R, Lodige B, Bulla M (1996) Renal insufficiency in the neonatal period. Clin Nephrol 46:54–58
Agras PI, Tarcan A, Baskin E, Cengiz N, Gurakan B, Saatci U (2004) Acute renal failure in the neonatal period. Ren Fail 26:305–309
Csaicsich D, Russo-Schlaff N, Messerschmidt A, Weninger M, Pollak A, Aufricht C (2008) Renal failure, comorbidity and mortality in preterm infants. Wien Klin Wochenschr 120:153–157
Gouyon JB, Guignard JP (2000) Management of acute renal failure in newborns. Pediatr Nephrol 14:1037–1044
Andreoli SP (2004) Acute renal failure in the newborn. Semin Perinatol 28:112–123
Toth-Heyn P, Drukker A, Guignard JP (2000) The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 14:227–239
Cataldi L, Leone R, Moretti U, De Mitri B, Fanos V, Ruggeri L, Sabatino G, Torcasio F, Zanardo V, Attardo G, Riccobene F, Martano C, Benini D, Cuzzolin L (2005) Potential risk factors for the development of acute renal failure in preterm newborn infants: a case-control study. Arch Dis Child Fetal Neonatal Ed 90:F514–F519
Vasarhelyi B, Toth-Heyn P, Treszl A, Tulassay T (2005) Genetic polymorphisms and risk for acute renal failure in preterm neonates. Pediatr Nephrol 20:132–135
Drukker A, Guignard JP (2002) Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr 14:175–182
Rodriguez-Soriano J, Aguirre M, Oliveros R, Vallo A (2005) Long-term renal follow-up of extremely low birth weight infants. Pediatr Nephrol 20:579–584
Spitzer A (1978) Renal physiology and functional development. In: Edelmann CM (ed) Pediatric kidney disease. Little Brown, Boston
Mathur NB, Agarwal HS, Maria A (2006) Acute renal failure in neonatal sepsis. Indian J Pediatr 73:499–502
Mortazavi F, Hosseinpour Sakha S, Nejati N (2009) Acute kidney failure in neonatal period. Iran J Kidney Dis 3:136–140
Askenazi DJ, Ambalavanan N, Goldstein SL (2009) Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol 24:265–274
Richardson DK, Corcoran JD, Escobar GJ, Lee SK (2001) SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr 138:92–100
Auron A, Mhanna MJ (2006) Serum creatinine in very low birth weight infants during their first days of life. J Perinatol 26:755–760
Miall LS, Henderson MJ, Turner AJ, Brownlee KG, Brocklebank JT, Newell SJ, Allgar VL (1999) Plasma creatinine rises dramatically in the first 48 hours of life in preterm infants. Pediatrics 104:e76
Chua AN, Sarwal MM (2005) Acute renal failure management in the neonate. Neoreviews 6:e369–e376
Cuzzolin L, Fanos V, Pinna B, di Marzio M, Perin M, Tramontozzi P, Tonetto P, Cataldi L (2006) Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol 21:931–938
Chevalier RL, Campbell F, Brenbridge AN (1984) Prognostic factors in neonatal acute renal failure. Pediatrics 74:265–272
Grylack L, Medani C, Hultzen C, Sivasubramanian K, Davitt MK, Jose P, Scanlon JW (1982) Nonoliguric acute renal failure in the newborn: a prospective evaluation of diagnostic indexes. Am J Dis Child 136:518–520
Karlowicz MG, Adelman RD (1995) Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol 9:718–722
Peruzzi L, Gianoglio B, Porcellini MG, Coppo R (1999) Neonatal end-stage renal failure associated with maternal ingestion of cyclo-oxygenase-type1 selective inhibitor nimesulide as tocolytic. Lancet 354:1615
Cuzzolin L, Dal Cere M, Fanos V (2001) NSAID-induced nephrotoxicity from the foetus to the child. Drug Saf 24:9–18
Aggarwal A, Kumar P, Chowdhary G, Majumdar S, Narang A (2005) Evaluation of renal functions in asphyxiated newborns. J Trop Pediatr 51:295–299
Casey BM, McIntire DD, Leveno KJ (2001) The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med 344:467–471
Itabashi K, Ohno T, Nishida H (2003) Indomethacin responsiveness of patent ductus arteriosus and renal abnormalities in preterm infants treated with indomethacin. J Pediatr 143:203–207
Alexander F, Chiu L, Kroh M, Hammel J, Moore J (2009) Analysis of outcome in 298 extremely low-birth-weight infants with patent ductus arteriosus. J Pediatr Surg 44:112–117, discussion 117
Dutta S, Narang A (2003) Enalapril-induced acute renal failure in a newborn infant. Pediatr Nephrol 18:570–572
Ng E, Taddio A, Ohlsson A (2003) Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev:CD002052
Moghal NE, Shenoy M (2008) Furosemide and acute kidney injury in neonates. Arch Dis Child Fetal Neonatal Ed 93:F313–F331
Fanos V, Antonucci R, Mussap M (2010) Drug induced nephrotoxicity in the newborn. The state of the art. Recent advances in clinical medicine. WSEAS press, pp 61–73
O’Neill JA Jr, Neblett WW 3rd, Born ML (1981) Management of major thromboembolic complications of umbilical artery catheters. J Pediatr Surg 16:972–978
Munoz-Arizpe R, Walsh RF, Edge W (1992) Obstructive aortic and renal thrombosis in the newborn–spontaneous recovery. Pediatr Nephrol 6:190–191
Ramasethu J (2008) Complications of vascular catheters in the neonatal intensive care unit. Clin Perinatol 35:199–222, x
Stapleton FB, Jones DP, Green RS (1987) Acute renal failure in neonates: incidence, etiology and outcome. Pediatr Nephrol 1:314–320
Satolli E (1994) Treatment of acute renal failure in newborn: the neonatological approach. Riv Ital Pediatr 20:328–330
Montini G, Barbieri P, Zaramella P (1995) Epidemiology of acute renal failure in the neonatal period. Ital J Pediatr 21:2–6
Abitbol CL, Bauer CR, Montane B, Chandar J, Duara S, Zilleruelo G (2003) Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol 18:887–893
Acknowledgments
This study was made possible by the Edward M. Chester MD Summer Scholars Program at MetroHealth Medical Center.
Funding and conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viswanathan, S., Manyam, B., Azhibekov, T. et al. Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol 27, 303–311 (2012). https://doi.org/10.1007/s00467-011-1977-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1977-8