Abstract
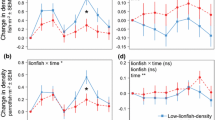
Although it is now recognized that mutualistic species are common and can have stable populations, the forces controlling their persistence are poorly understood. To better understand the mechanisms that impact the stability of obligate mutualists, I conducted several field experiments within a sandy coral reef lagoon in Moorea, French Polynesia that manipulated densities of fish (gobies) that interact mutualistically with shrimp. Obligate, mutualistic partnerships of gobies and shrimp are common on Indo-Pacific coral reefs and have been shown previously to interact as follows: shrimp construct burrows in which both species reside, and gobies warn shrimp of predators through tactile communication. Augmentation of gobies by up to 100% above ambient densities within 9 m2 plots produced no change in overall density of gobies or shrimp because gobies competed intraspecifically for a limited number of shrimp burrows and smaller gobies were outcompeted by larger individuals. I used predators to assess the impact of goby removal on the stability of goby and shrimp populations. First, although surveys taken throughout the lagoon revealed no relationship between goby and predator densities, predators correlated negatively with the proportion of adult gobies and positively with the proportion of small gobies paired with large shrimp. Second, experimental augmentation of predators resulted in a dramatic reduction of adult gobies within predator-addition plots, but had no impact on overall densities as immigrants rapidly replaced the missing adult gobies. Furthermore, goby turnover resulted in an increase in the proportion of small gobies paired with large shrimp because body sizes of gobies and shrimp in a burrow were similar prior to predator introduction, and predators apparently had a greater impact on gobies than shrimp. The mechanisms that prevent expansion (intraspecific competition) and collapse (immigration) of goby-shrimp populations likely contribute to local-scale stability of mutualistic populations in other terrestrial and aquatic environments.





Similar content being viewed by others
References
Agrawal AA, Karban R (1997) Domatia mediate plant-anthropod mutualism. Nature 387:562–563
Amarasekare P (2004) Spatial dynamics of mutualistic interaction. J Anim Ecol 73:128–142
Billick I, Tonkel K (2003) The relative importance of spatial versus. temporal variability in generating a conditional mutualism. Ecology 84:289–295
Boucher DH, James S, Keeler KH (1982) The ecology of mutualism. Annu Rev Ecol Syst 13:315–347
Brenton LM, Addicott JF (1992) Density-dependent mutualism in an aphid-ant interaction. Ecology 73:2175–2180
Buston P (2003) Forcible eviction and prevention of recruitment in the clown anemonefish. Behav Ecol 14:576–582
Connor RC (1995) The benefits of mutualism: a conceptual framework. Biol Rev 70:427–457
Cummins RA (1979) Ecology of Gobiid fishes associated with Alpheid shrimps. Ph.D. Dissertation, University of Sydney
Dean AD (1983) A simple model of mutualism. Am Nat 121:409–417
Dickman CR (1992) Commensal and mutualistic interactions among terrestrial vertebrates. Trends Ecol Evol 7:194–197
Hernandez M-J, Barrada I (2003) Variation in the outcome of population interactions: bifurcations and catastrophes. J Math Biol 46:571–594
Hobbs J-PA, Munday PL (2004) Intraspecific competition controls spatial distribution and social organization of the coral-dwelling goby Gobiodon histrio. Mar Ecol Prog Ser (in press)
Holland JN, DeAngelis DL, Bronstein JL (2002) Population dynamics and mutualism: functional responses of benefits and costs. Am Nat 159:231–244
Hutson V, Law R, Lewis D (1985) Dynamics of ecologically obligate mutualisms—effects of spatial diffusion on resilience of the interacting species. Am Nat 126:445–448
Jones GP, Milicich MJ, Emslie MJ, Lunow C (1999) Self-recruitment in a coral reef fish population. Nature 402:802–804
Karplus I (1987) The association between gobiid fishes and burrowing alpheid shrimps. Ocean Mar Biol 25:507–562
Karplus I, Szlep R, Tsurnamal M (1974) The burrows of alpheid shrimp associated with Gobiid fish in the Northern Red Sea. Mar Biol 24:259–268
Karplus I, Tsurnamal M, Szlep R, Algom D (1979) Film analysis of the tactile communication between Cryptocentrus steinitzi (Pisces, Gobiidae) and Alpheus purpurilenticularis (Crustacea, Alpheidae). Z Tierpsychol 49:337–351
May RM (1973) Qualitative stability in model ecosystems. Ecology 54:638–641
May RM (1982) Mutualistic interactions among species. Nature 296:803–804
Meyer JL, Schultz ET, Helfman GS (1983) Fish schools—an asset to corals. Science 220:1047–1049
Mora C, Sale PF (2002) Are populations of coral reef fish open or closed? Trends Ecol Evol 17:422–428
Morales MA (2000) Mechanisms and density dependence of benefit in an ant-membracid mutualism. Ecology 81:482–489
Murdoch WW (1994) Population regulation in theory and practice. Ecology 75:271–287
Norton AP, English-Loeb G, Gadoury D, Seem RC (2000) Mycophagous mites and foliar pathogens: leaf domatia mediate tritrophic interactions in grapes. Ecology 81:490–499
Polunin NVC, Lubbock R (1977) Prawn-associated gobies (Teleostei: Gobiidae) from the Seychelles, Western Indian Ocean: systematics and ecology. J Zool London 183:63–101
Pulliam R (1988) Sources, sinks and population regulation. Am Nat 132:652–661
Schmitt RJ, Holbrook SJ (2003) Mutualism can mediate competition and promote coexistence. Ecol Lett 6:898–902
Sponaugle S, Cowen RK (1994) Larval durations and recruitment patterns of 2 Caribbean gobies (Gobiidae)—Contrasting early-life histories in demersal spawners. Mar Biol 120:133–143
Stachowicz JJ (1999) Mutualism, facilitation, and the structure of ecological communities. BioScience 51:235–246
Stachowicz JJ, Hay ME (1999) Mutualism and coral persistence: the role of herbivore resistance to algal chemical defense. Ecology 80:2085–2101
Thompson AR (2003) Population ecology of marine mutualists. Ph.D. Dissertation, University of California, Santa Barbara
Thompson AR (2004) Habitat and mutualism affect the distribution and abundance of a shrimp-associated goby. Mar Freshwater Res 55:105–113
Vandermeer JH, Boucher DH (1978) Varieties of mutualistic interactions in population models. J Theor Biol 74:549–558
Williamson MH (1972) The analysis of biological populations. Edward Arnold, London
Yangisawa Y (1982) Social behaviour and mating system of the gobiid fish Amblyeleotris japonica. Jpn J Ichth 28:401–422
Yangisawa Y (1984) Studies on the interspecific relationship between gobiid fish and snapping shrimp. II. Life history and pair formation of snapping shrimp Alpheus bellulus. Publ Seto Mar Biol Lab 29:93–116
Zacherl DC (2003) Trace elemental fingerprinting of gastropod statoliths to study larval dispersal trajectories. Mar Ecol Prog Ser 248:297–303
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgements
I would like to thank A. Anker, S. Cooper, S. Holbrook, I. Karplus, B. Larson, P. Munday, R. Nisbet and R. Schmitt for critical discussion, N. Davies and B. Williamson for technical assistance, and S. Ferse, D. Geiger, M. Schmitt, S. Schellenberg, C. Thacker, B. Wolcott, and, especially, J. White for assistance in the field. Funding for this work was provided by R.T.G. and G.R.T. Programs in Spatial Ecology (NSF BIR94-13141 and NSF GER93-54870, both to W. Murdoch) and grants by the National Science Foundation to R Schmitt and S. Holbrook (OCE99-10677) and R. Nisbet (DEB01-08450). This paper is contribution #112 of the UC Berkeley Richard B. Gump South Pacific Research Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, A.R. Dynamics of demographically open mutualists: immigration, intraspecific competition, and predation impact goby populations. Oecologia 143, 61–69 (2005). https://doi.org/10.1007/s00442-004-1775-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1775-0