Abstract
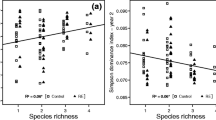
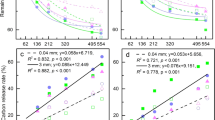
Plant species composition is a potentially important source of variation in soil processes, including decomposition rates. We compared litter decomposition in two common and compositionally distinct tundra vegetation types in the northern foothills of the Brooks Range, Alaska: moist acidic tundra (soil pH 3–4), which occurs primarily on older landscapes, and moist non-acidic tundra (soil pH 6–7), which occurs primarily on landscapes with a more recent history of glaciation and has higher graminoid and forb abundance and lower woody shrub abundance than acidic tundra. To separate the influence of plant community composition from that of the soil environment, we decomposed the same nine substrates at a moist acidic and a moist non-acidic site located less than 2 km apart. Substrates included leaf litter of the dominant species in each growth form (graminoid, deciduous shrub, evergreen shrub, forb, moss) as well as woody stems of the deciduous shrub Betula nana. Then, we estimated above-ground community-level decomposition by weighting the decay rate of each species in the community by its proportional contribution to overall above-ground net primary production (ANPP). In contrast to our expectations, community-level decomposition rates estimated using the site-average decay rate for each substrate were similar between the two sites, likely because growth forms differed little in their leaf litter decay. By contrast, when site-specific decay rates were used to estimate community-level decomposition, it was nearly twice as fast at the older, moist acidic tundra site because most substrates decayed faster at that site, indicating a more favorable environment for decomposition in acidic tundra. Site differences in soil moisture and temperature could not explain site differences in decomposition. However, higher soil N availability at the moist acidic tundra may have contributed to faster decomposition since, in a separate experiment, fertilization with N stimulated decomposition of a common substrate at both sites. In addition, lower pH in acidic tundra may promote greater abundance of soil fungi, perhaps explaining faster decomposition rates at that site. In summary, the large differences in plant species composition between moist acidic and non-acidic tundra are likely to not contribute to site differences in decomposition. Nevertheless, decomposition is much more rapid in moist acidic tundra. Thus, landscape age and associated differences in soil pH and nutrient availability are important sources of variation in decomposition rate in upland Alaskan tundra.





Similar content being viewed by others
References
Baath E, Arnebrandt K (1994) Growth rate and response of bacterial communities to pH in limed and ash treated forest soils. Soil Biol Biochem 26:995–1001
Bevington PR (1969) Data reduction and error analysis for the physical sciences. McGraw-Hill, New York
Bockheim JG, Walker DA, Everett LR, Shiklomanov NI (1998) Soils and cryoturbation in moist nonacidic and acidic tundra in the Kuparuk River Basin, Arctic Alaska, USA. Arct Alpine Res 30:166–174
Bret-Harte MS, Shaver GR, Chapin FS III (2002) Primary and secondary stem growth in arctic shrubs: implications for community response to environmental change. J Ecol 90:251–267
Chan KY, Heenan DP (1999) Lime-induced loss of soil organic carbon and effect on aggregate stability. Soil Sci Soc Am J 63:1841–1844
Coulson JC, Butterfield J (1978) An investigation of the biotic factors determining the rates of plant decomposition on a blanket bog. J Ecol 66:631–650
Dowding P, Widden P (1974) Some relationships between fungi and their environment in tundra regions. In: Holding AJ, Heal OW, MacLean SF Jr, Flanagan PW (eds) Soil organisms and decomposition in tundra. Tundra Biome Steering Committee, Stockholm, pp 123–150
Evans RD, Rimer R, Sperr (2001) Exotic plant invasion alters nitrogen dynamics in an arid grassland. Ecol Appl 11:1301–1310
Finzi AC, Canham CD, Van Breemen N (1998a) Canopy tree-soil interactions within temperate forests: species effects on pH and cations. Ecol Appl 8:447–454
Finzi AC, Van Breemen N, Canham CD (1998b) Canopy tree-soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol Appl 8:440–446
Gough L, Hobbie SE (2003) Responses of moist non-acidic arctic tundra to altered environment: productivity, biomass and species richness. Oikos 103:204–216
Gough L, Shaver GR, Carroll J, Royer DL, Laundre JA (2000) Vascular plant species richness in Alaskan arctic tundra: the importance of soil pH. J Ecol 88:54–66
Hamilton TD (2002) Glacial geology of the Toolik Lake and Upper Kuparuk River region. In: Walker DA (ed) University of Alaska Fairbanks, Institute of Arctic Biology, Fairbanks
Heal OW, French DD (1974) Decomposition of organic matter in tundra. In: Holding AJ, Heal OW, Maclean SF, Flanagan PW (eds) Soil organisms and decomposition in tundra. Tundra Biome Steering Committee, Stockholm, pp 279–310
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Hobbie SE, Chapin FS III (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35:327–338
Hobbie SE, Chapin FS III (1998) The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to temperature manipulation. Ecology 79:1526–1544
Hobbie SE, Gough L (2002) Foliar and soil nutrients in tundra on glacial landscapes of contrasting ages in northern Alaska. Oecologia 131:453–463
Hobbie SE, Miley TA, Weiss MS (2002) Carbon and nitrogen cycling in soils from different glacial surfaces in northern Alaska. Ecosystems 5:761–774
Hooper DU, Vitousek PM (1998) Effects of plant composition and diversity on nutrient cycling. Ecol Monogr 68:121–149
Lovett GM, Rueth H (1999) Soil nitrogen transformations in beech and maple stands along a nitrogen deposition gradient. Ecol Appl 9:1330–1344
Lovett GM, Weathers KC, Arthur MA (2002) Controls of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 5:712–718
Lovett GM, Weathers KC, Arthur MA, Schultz JC (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308
Mack MC, D’Antonio DM, Ley RE (2001) Alteration of ecosystem nitrogen dynamics by exotic plants: a case study of C4 grasses in Hawaii. Ecol Appl 11:1323–1335
Muneer M, Oades JM (1989a) The role of Ca-organic interactions in soil aggregate stability. I. Laboratory studies with 14 C-glucose, CaCO3 and CaSO42H2O. Aust J Soil Res 27:389–399
Muneer M, Oades JM (1989b) The role of Ca-organic interactions in soil aggregate stability. II. Field studies with 14C-labelled straw, CaCO3 and CaSO42H2O. Aust J Soil Res 27:401–409
Munter RC, Grande RA (1981) Plant tissue and soil extract analysis by ICP-AES. In: Barnes RM (ed) Developments in atomic plasma spectrochemical analysis. Heydon, Philadephia, pp 653–673
Neale SP, Shah Z, Adams WA (1997) Changes in microbial biomass and nitrogen turnover in acidic organic soils following liming. Soil Biol Biochem 29:1463–1474
Painter TG (1991) Lindow Man, Tollund Man, and other peat-bog bodies: the preservative and antimicrobial action of sphagnan, a reactive glycuronoglycan with tanning and sequestering properties. Carbohydr Polymers 15:123–142
Robinson CH (2002) Controls on decomposition and soil nitrogen availability at high latitudes. Plant Soil 242:65–81
Robinson CH, Wookey PA, Parsons AN, Potter JA, Callaghan TV, Lee JA, Press MC, Welker JM (1995) The response of plant litter decomposition and nitrogen mineralisation to simulated environmental change in a high Arctic polar desert and subarctic dwarf shrub heath. Oikos 74:503–512
Scott NA, Binkley D (1997) Foliage litter quality and annual net N mineralization: comparison across North American forest sites. Oecologia 111:151–159
Scott NA, Saggar S, McIntosh PD (2001) Biogeochemical impact of Hieracium invasion in New Zealand’s grazed tussock grasslands: sustainability implications. Ecol Appl 11:1311–1322
Shaver GR, Chapin FS III (1991) Production:biomass relationships and element cycling in contrasting arctic vegetation types. Ecol Monogr 61:1–31
Shaver GR et al (2001) Species composition interacts with fertilizer to control long-term change in tundra productivity. Ecology 82:3162–3181
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell, Oxford
Van Cleve K (1974) Organic matter quality in relation to decomposition. In: Holding AJ, Heal OW, Maclean SF, Flanagan PW (eds) Soil organisms and decomposition in tundra. Tundra Biome Steering Committee, Stockholm, pp 311–324
Van Soest PJ (1994) Nutritional ecology of the ruminant, 2nd edn. Cornell University Press, Ithaca, N.Y.
Vinton MA, Burke IC (1995) Interactions between individual plant species and soil nutrient status in shortgrass steppe. Ecology 76:1116–1133
Vinton MA, Burke IC (1997) Contingent effects of plant species on soils along a regional moisture gradient in the Great Plains. Oecologia 110:393–402
Vitousek PM, Walker LR (1989) Biological invasion by Myrica faya in Hawai’i: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59:247–265
Walker DA (1998) The Geobotanical Atlas of the Toolik Lake and Kuparuk River Region, Alaska. http://www.geobotany.uaf.edu/arcticgeobot/index.html
Walker MD, Walker DA, Auerbach NA (1994) Plant communities of a tussock tundra landscape in the Brooks Range Foothills, Alaska. J Veg Sci 5:843–866
Walker DA, Auerbach NA, Shippert MM (1995) NDVI, biomass, and landscape evolution of glaciated terrain in northern Alaska. Polar Rec 31:169–178
Walker DA et al (1998) Energy and trace-gas fluxes across a soil pH boundary in the Arctic. Nature 394:469–472
Wedin DA, Tilman D (1990) Species effects on nitrogen cycling: a test with perennial grasses. Oecologia 84:433–441
Acknowledgements
We thank Tiffany Miley, Angela Moline, Chinelo Njaka, and Marissa Weiss for assistance in the field or laboratory and Merritt Turetsky and two anonymous reviewers for comments on the manuscript. We also thank Stan Harpole and Janneke HilleRisLambers for useful discussion. We are grateful to the Toolik Lake Field Research Station and the Arctic LTER for logistic support. This research was supported by a collaborative grant from the National Science Foundation (OPP-9902695 to S. E. Hobbie and OPP-9902721 to L. Gough). Fertilizer manipulations were initiated and maintained with support from the National Science Foundation to the Arctic LTER (DEB-9810222).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hobbie, S.E., Gough, L. Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia 140, 113–124 (2004). https://doi.org/10.1007/s00442-004-1556-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1556-9