Abstract
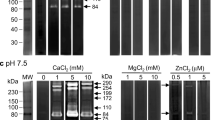
Acid phosphatases are putative virulence factors in different pathogenic microorganisms. Acid phosphatases can also inhibit the respiratory burst of human neutrophils. In Cryptosporidium parvum, a protozoan parasitic, the study of enzymes is limited. In this paper, we report the presence of a membrane-bound acid phosphatase activity in C. parvum oocysts. The enzymatic activity was inhibited by protein tyrosine phosphatase inhibitors such as sodium orthovanadate, ammonium molybdate, and sodium tungstate and was not affected by protein serine/threonine phosphatase inhibitors such as okadaic acid and calyculin. Antibodies against the catalytic domain of human placental PTPase 1B cross-reacted with two molecules of 30 and 31 kDa present in membrane fraction of a Cryptosporidium oocyst homogenate. This is the first demonstration of acid phosphatase activity in Cryptosporidium.


Similar content being viewed by others
References
Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier A, Dear PH, Konfortov BA, Spriggs HF, Iyer L, Anantharaman V, Aravind L, Kapur V (2004) Complete genome sequence of Apicomplexan, Cryptosporidium parvum. Science 304:441–445
Aguirre-García MM, Cerbón J, Talamás-Rohana P (2000) Purification and properties of an acid phosphatase from Entamoeba histolytica HM-1:IMSS. Int J Parasitol 30:585–591
Aguirre-García MM, Anaya-Ruiz M, Talamás-Rohana P (2003) Membrane-bound acid phosphatase (MAP) from Entamoeba histolytica has phosphotyrosine phosphatase activity and disrupts the actin cytoskeleton of host cells. Parasitology 126:195–202
Aguirre-García MM, Escalona-Montaño AR, Bakalara N, Pérez-Torres A, Gutiérrez-Kobeh L, Becker I (2006) Leishmania major: detection of membrane-bound protein tyrosine phosphatase. Parasitology 5:1–9
Arrowood MJ, Sterling CR (1987) Isolation of Cryptosporidium oocysts and sporozoites using discontinous sucrose and isopycnic percoll gradient. J Parasitol 73:314–319
Baca OG, Roman MJ, Glew RH, Christner RF, Buhler JE, Aragon AS (1993) Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun 61:4232–4239
Bakalara N, Seyfang A, Baltz T, Davis C (1995) Trypanosoma brucei and Trypanosoma cruzi: Life cycle-regulated protein tyrosine phosphatase activity. Exp Parasitol 81:302–312
Bliska JB, Black DS (1995) Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect Immun 63:681–685
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254
Chen XM, Huang BQ, Splinter PL, Cao H, Zhu G, Mcniven MA, Larusso NF (2003) Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-Src. Gastroenterology 125:216–228
De Vinney R, Steele-Mortimer O, Finlay BB (2000) Phosphatases and kinases delivered to the host cell by bacterial pathogens. Trends Microbiol 8:29–33
DuPont H, Chapell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski DW (1995) The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 332:855–859
Entrala E, Mascaro C, Barret J (1997) Anti-oxidant enzymes in Cryptosporidium parvum oocysts. Parasitology 114:13–17
Gee KR, Sun W, Bhalgat MK, Upson RH, Klaubert D, Latham K, Haugland RP (1999) Fluorogenic substrates based on fluorinated umbelliferones for continuous assays of phosphatases and β-Galactosidases. Ann Biochem 273:41–48
Glew HR, Czuczman MS, Diven WF, Berens RL, Pope MT, Katsoulis DE (1982) Partial purification and characterization of particulate acid phosphatase of Leishmania donovani promastigotes. Comp Biochem Physiol 72B:581–590
Green SP, Hartland EL, Robins-Browne RM, Phillips WA (1995) Role of YopH in the suppression of tyrosine phosphorylation and respiratory burst activity in murine macrophages infected with Yersinia enterocolitica. J Leukoc Biol 57:972–977
Hardie DG (1993) Use of protein phosphatase inhibitors in intact cells. In: Hardie DG (ed) Protein phosphorylation: a practical approach. IRL, Oxford, pp 109–119
Koul A, Choidas A, Treder M, Tyagi A, Drlica K, Singh Y, Ullrich A (2000) Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. J Bacteriol 182:5425–5432
Li YP, Curley G, Lopez M, Chavez M, Glew R, Aragon A, Kumar H, Baca OG (1996) Protein-tyrosine phosphatase activity of Coxiella burnetii that inhibits human neutrophils. Acta Virol 40:263–272
Menz B, Winter G, Ilg T, Lottspeich F, Overath P (1991) Purification and characterization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol Biochem Parasitol 47:101–108
Nagakura K, Tachibana H, Kaneda Y (1985) Alteration of the cell surface acid phosphatase concomitant with the morphological transformation in Trypanosoma cruzi. Comp Biochem Physiol 81B:815–817
Priest JW, Xie L, Arrowood MJ, Lammie PJ (2001) The immunodominant 17 kDa antigen from Cryptosporidium parvum is glycosylphosphatidylinositol-anchored. Mol Biochem Parasitol 13:117–126
Reilly TJ, Baron GS, Nano FE, Kuhlenschmidt MS (1996) Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J Biol Chem 271:10973–10983
Remaley AT, Kuhns DB, Basford RE, Glew, RH, Kaplan S (1984) Leishmanial phosphatase blocks neutrophil \( {\text{O}}^{ - }_{2} \) production. J Biol Chem 259:11173–11175
Saha AK, Dowling JN, Lamarco KL, Das S, Remaley AT, Olomu N, Pope MT, Glew RH (1985) Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys 243:150–160
Schmid B, Wimmer M, Tag C, Hoffmann R, Hofer HW (1996) Protein phosphotyrosine phosphatases in Ascaris suum muscle. Mol Biochem Parasitol 77:183–192
Shibata KI, Noda M, Sawa Y, Watanabe T (1994) Acid phosphatase purified from Mycoplasma fermentans has protein tyrosine phosphatase-like activity. Infect Immun 62:313–315
Zhang Z-Y, Dixon JE (1994) Protein tyrosine phosphatases: mechanism of catalysis and substrate specificity. In: Meister A (ed) Advances in enzymology. Wiley, New York, pp 68:1–36
Acknowledgment
We thank Alma Escalona-Montaño and Lily Carlin for excellent technical assistance. Dr. Patricia Talamás Rohana for critically reading of the manuscript. This work was supported by grants R01 AI 41735-01, FD-R-001621, and 45052-M from CONACyT, México.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguirre-García, M.M., Okhuysen, P.C. Cryptosporidium parvum: identification and characterization of an acid phosphatase. Parasitol Res 101, 85–89 (2007). https://doi.org/10.1007/s00436-006-0457-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0457-8