Abstract
Purpose
Panitumumab monotherapy is approved for KRAS wild-type (WT) metastatic colorectal cancer (mCRC) progressing after standard chemotherapy. This study evaluated first-line panitumumab plus FOLFIRI in patients with mCRC.
Methods
In this phase II, single-arm study, panitumumab (6 mg/kg) and FOLFIRI [irinotecan (180 mg/m2) and leucovorin (400 mg/m2) followed by a 5-fluorouracil 400 mg/m2 bolus and a 2,400–3,000 mg/m2 continuous infusion] were administered every 14 days until progression. Data were analysed descriptively overall and by tumour KRAS status.
Results
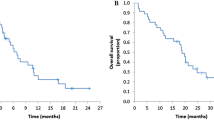
KRAS data were available for 145/154 (94%) patients: 59% KRAS WT and 41% mutant (MT); mean follow-up was 39.5 versus 35.8 weeks, respectively. Objective responses occurred in 49% of patients: 56% versus 38% in the KRAS WT versus MT groups [(18% difference (95% CI 1–35%); odds ratio 2.1 (95% CI 1.0–4.4)]; median duration of response was 13.0 versus 7.4 months. More patients in the WT group underwent R0 resection (8% vs. 5%); median progression-free survival also favoured this group (8.9 vs. 7.2 months). The most common adverse events (any grade) were integument toxicities (98%), diarrhoea (79%) and stomatitis/oral mucositis (51%).
Conclusions
As expected, consistently favourable efficacy was observed in patients with KRAS WT versus MT tumours receiving first-line panitumumab plus FOLFIRI treatment.



Similar content being viewed by others
References
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634. doi:10.1200/JCO.2007.14.7116
Amgen Inc (2010) Vectibix® prescribing information http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf
Amgen Ltd (2009) Vectibix® (panitumumab) summary of product characteristics http://www.emc.medicines.org.uk/medicine/20528/SPC/Vectibix/
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F et al (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648. doi:10.1158/0008-5472.CAN-06-4158
Bokemeyer C, Bondarenko I, Makhson A et al (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671. doi:10.1200/JCO.2008.20.8397
Cohenuram M, Saif MW (2007) Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs 18:7–15. doi:10.1097/CAD.0b013e32800feecb
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345. doi:10.1056/NEJMoa033025
De Roock W, Piessevaux H, De Schutter J et al (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515. doi:10.1093/annonc/mdm496
Di Fiore F, Blanchard F, Charbonnier F et al (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96:1166–1169. doi:10.1038/sj.bjc.6604451
Dolgin E (2009) FDA narrows drug label usage. Nature 460:1069. doi:10.1038/4601069a
Douillard JY, Cunningham D, Roth AD et al (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041–1047. doi:10.1016/S0140-6736(00)02034-1
Douillard JY, Siena S, Cassidy J et al (2010) Randomized, phase 3 study (PRIME) of panitumumab with FOLFOX4 versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 28:4697–4705. doi:10.1200/JCO.2009.27.4860
European Medicines Agency (2009a) Erbitux® (cetuximab) prescribing information http://www.emea.europa.eu/humandocs/PDFs/EPAR/erbitux/H-558-PI-en.pdf
European Medicines Agency (2009b) Vectibix® (panitumumab) prescribing information http://www.emea.europa.eu/humandocs/PDFs/EPAR/vectibix/H-741-PI-en.pdf
European Medicines Agency (2011) Committee for Medicinal Products for Human Use (CHMP). Summary of opininin (post-authorisation)—Vectibix (panitumumab) 2011 http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000741/WC500107994.pdf
Freeman DJ, Juan T, Reiner M et al (2008) Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer 7:184–190. doi:10.3816/CCC.2008.n.024
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol 25:4779–4786. doi:10.1200/JCO.2007.11.3357
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342. doi:10.1056/NEJMoa032691
Kari C, Chan TO, Rocha de Quadros M, Rodeck U (2003) Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res 63:1–5
Koo DH, Lee JL, Kim TW et al (2007) A Phase II study of cetuximab (Erbitux) plus FOLFIRI for irinotecan and oxaliplatin-refractory metastatic colorectal cancer. J Korean Med Sci 22(Suppl):S98–S103
Lièvre A, Bachet JB, Le Corre D et al (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995
National Cancer Institute Cancer Therapy Evaluation Program [CTEP] (2005) Common toxicity criteria for adverse events v3.0 (CTCAE) http://ctep.cancer.gov/protocolDevelopment/electronic_applicationa/docs/ctcaev3.pdf
National Cancer Institute Cancer Therapy Evaluation Program [CTEP] (2006) Common terminology criteria for adverse events (CTCAE), Version 3.0, DCTD, NCI, NIH, DHHS http://www.ctep.cancer.gov
Peeters M, Price T, Cervantes A et al (2010) Randomized phase 3 study of panitumumab with FOLFIRI versus FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706–4713
Raoul JL, Van Laethem JL, Peeters M et al (2009) Cetuximab in combination with irinotecan/5-fluorouracil/folinic acid (FOLFIRI) in the initial treatment of metastatic colorectal cancer: a multicentre two-part phase I/II study. BMC Cancer 9:112. doi:10.1186/1471-2407-9-112
Saltz LB, Cox JV, Blanke C et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208. doi:10.1200/JCO.2004.10.182
Santini D, Loupakis F, Vincenzi B et al (2008) High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist 13:1270–1275. doi:10.1634/theoncologist.2008-0181
Segaert S, Van Cutsem E (2005) Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16:1425–1433. doi:10.1093/annonc/mdi279
Sobrero AF, Maurel J, Fehrenbacher L et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319. doi:10.1200/JCO.2007.13.1193
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216. doi:10.1093/jnci/92.3.205
Tournigand C, André T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237. doi:10.1200/JCO.2004.05.113
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664. doi:10.1200/JCO.2006.08.1620
Van Cutsem E, Köhne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417. doi:10.1056/NEJMoa0805019
Yen LC, Yeh YS, Chen CW et al (2009) Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res 15:4508–4513. doi:10.1158/1078-0432.CCR-08-3179
Acknowledgments
This work was supported by Amgen (Europe) GmbH, Zug, Switzerland. Medical writing support [funded by Amgen (Europe) GmbH] provided by Dawn Batty (Bioscript Stirling Ltd); manuscript preparation assistance provided by Emma Thomas (Amgen). Pamela Ward and David Eaton: study management; Marco Schupp: study design/initiation; Karin Blakolmer: critical review of the data/manuscript (all Amgen employees).
Conflict of interest
CHK has acted as a consultant/advisor to Amgen Ltd, Merck KG Damstadt & Roche Ltd; HL has acted as a consultant/advisor for Amgen Ltd & Roche Ltd; RH, JT & MK have received honoraria from Amgen Ltd, JT has also received research funding and MK has also acted as an advisor for this company; EG & LDC are employees of Amgen Ltd and also own shares in this company.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Köhne, CH., Hofheinz, R., Mineur, L. et al. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol 138, 65–72 (2012). https://doi.org/10.1007/s00432-011-1061-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1061-6