Abstract
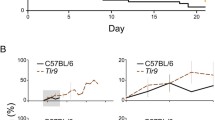
The contribution of the Toll-like receptor (TLR) cascade to the pathogenesis of cerebral malaria (CM) is controversially discussed. TLR2 and TLR9 were reported to be involved in the induction of CM in a study while recently TLR signaling was shown to be dispensable for the development of CM. Using Plasmodium berghei ANKA (PbA) infection of mice as a model of CM, we demonstrate here that the induction of CM is independent of TLR2, 4 and 9. Using triple TLR2/4/9-deficient mice, we exclude synergistic effects between the single TLRs that have been previously implicated with malaria pathology. In conclusion, this study shows that the activation of the innate immune response and the development of CM is not dependent on the engagement of TLR2/4/9.




Similar content being viewed by others

References
Severe falciparum malaria. World Health Organization, communicable diseases cluster. Trans R Soc Trop Med Hyg 2000; 94 (Suppl 1):S1–90
MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA (1985) Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathology 119:385–401
Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415:673–679
Idro R, Jenkins NE, Newton CR (2005) Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 4:827–840
Engwerda C, Belnoue E, Gruner AC, Renia L (2005) Experimental models of cerebral malaria. Curr Top Microbiol Immunol 297:103–143
Hearn J, Rayment N, Landon DN, Katz DR, de Souza JB (2000) Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect Immun 68:5364–5376
Neill AL, Hunt NH (1992) Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology 105(Pt 2):165–175
Grau GE, Bieler G, Pointaire P, De Kossodo S, Tacchini-Cotier F, Vassalli P, Piguet PF, Lambert PH (1990) Significance of cytokine production and adhesion molecules in malarial immunopathology. Immunol Lett 25:189–194
Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P (1987) Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210–1212
Grau GE, Frei K, Piguet PF, Fontana A, Heremans H, Billiau A, Vassalli P, Lambert PH (1990) Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med 172:1505–1508
Finley RW, Mackey LJ, Lambert PH (1982) Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J Immunol 129:2213–2218
Belnoue E, Kayibanda M, Vigario AM, Deschemin JC, van Rooijen N, Viguier M, Snounou G, Renia L (2002) On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol 169:6369–6375
Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH (1986) L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol 137:2348–2354
Yanez DM, Manning DD, Cooley AJ, Weidanz WP, van der Heyde HC (1996) Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol 157:1620–1624
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Zhu J, Krishnegowda G, Gowda DC (2005) Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem 280:8617–8627
Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S (2005) Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med 201:19–25
Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT (2007) Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci USA 104:1919–1924
Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC (2005) Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 280:8606–8616
Mockenhaupt FP, Cramer JP, Hamann L, Stegemann MS, Eckert J, Oh NR, Otchwemah RN, Dietz E, Ehrhardt S, Schroder NW, Bienzle U, Schumann RR (2006) Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. J Commun Dis 38:230–245
Adachi K, Tsutsui H, Kashiwamura S, Seki E, Nakano H, Takeuchi O, Takeda K, Okumura K, Van Kaer L, Okamura H, Akira S, Nakanishi K (2001) Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J Immunol 167:5928–5934
Coban C, Ishii KJ, Uematsu S, Arisue N, Sato S, Yamamoto M, Kawai T, Takeuchi O, Hisaeda H, Horii T, Akira S (2007) Pathological role of Toll-like receptor signaling in cerebral malaria. Int Immunol 19:67–79
Togbe D, Schofield L, Grau GE, Schnyder B, Boissay V, Charron S, Rose S, Beutler B, Quesniaux VF, Ryffel B (2007) Murine cerebral malaria development is independent of toll-like receptor signaling. Am J Pathol 170:1640–1648
Yasuda K, Yu P, Kirschning CJ, Schlatter B, Schmitz F, Heit A, Bauer S, Hochrein H, Wagner H (2005) Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol 174:6129–6136
Tachado SD, Gerold P, McConville MJ, Baldwin T, Quilici D, Schwarz RT, Schofield L (1996) Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J Immunol 156:1897–1907
Schofield L, Novakovic S, Gerold P, Schwarz RT, McConville MJ, Tachado SD (1996) Glycosylphosphatidylinositol toxin of plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol 156:1886–1896
Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A (2006) Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol 177:3515–3519
Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM (2007) TLR2 synergizes with both TLR4, TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol 245(2): 103–110
Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150
Acknowledgments
This work was supported by the DFG grant JA 1451 to T.J. We thank Iris Gaworski for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lepenies, B., Cramer, J.P., Burchard, G.D. et al. Induction of experimental cerebral malaria is independent of TLR2/4/9. Med Microbiol Immunol 197, 39–44 (2008). https://doi.org/10.1007/s00430-007-0057-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-007-0057-y