Abstract
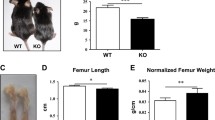
Parathyroid hormone-related protein (PTHrP) null mutant mice were analyzed to investigate an additional role for PTHrP in cell differentiation. We found ectopic cartilage formation in the mandibular coronoid process in newborn mice. While many previous studies involving PTHrP gene knockout mouse have shown that the cartilage in various regions becomes smaller, this is the first report showing an "increase" of cartilage volume. Investigations of mandibular growth using normal mice indicated that coronoid secondary cartilage never formed from E 15 to d 4, but small amount of cartilage temporally formed at d 7, and this also applies to PTHrP-wild type mice. Therefore, PTHrP deficiency consequently advanced the secondary cartilage formation, which is a novel role of PTHrP in chondrocyte differentiation. In situ hybridization of matrix proteins showed that this coronoid cartilage had characteristics of the lower hypertrophic cell zone usually present at the site of endochondral bone formation and/or "chondroid bone" occasionally found in distraction osteogenesis. In addition, the coronoid process in the PTHrP-deficient mouse also showed abnormal expansion of bone marrow and an increase in the number of multinucleated osteoclasts, an indication of abnormal bone modeling. These results indicate that PTHrP is involved in bone modeling as well as in chondrocyte differentiation. In situ hybridization of matrix protein mRNAs in the abnormal mandibular condylar cartilage revealed that this cartilage was proportionally smaller, supporting previous immunohistochemical results.






Similar content being viewed by others
References
Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC (1994) Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol 126:1611-1623
Arai N, Ohya K, Ogura H (1993) Osteopontin mRNA expression during bone resorption: an in situ hybridization study of induced ectopic bone in the rat. J.Bone Miner Res 22:129-145
Arai N, Ohya K, Kasugai S, Shimokawa H, Oida S, Oura H, Amagasa T (1995) Expression of bone sialoprotein mRNA during bone formation and resorption induced by colchicine in rat tibial bone marrow cavity. J Bone Miner Res 10:1209-1217
Beresford WA (1981) Chondroid bone, Secondary cartilage and Metaplasia. Urban& Schwarzenberg, Baltimore, pp. 23-65
Chen J, Shapiro HS, Wrana JL, Reimers S, Heersche JNM, Sodek J (1991) Localization of bone sialoprotein (BSP) expression to sites of mineralized tissue formation in fetal rat tissues by in situ hybridization. Matrix 11:133-143
Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T (1999) In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J Anat 195:321-329
Ishii-Suzuki M, Suda N, Yamazaki K, Kuroda T, Senior PV, Beck F, Hammond VE (1999) Differential responses to parathyroid hormone-related protein (PTHrP) deficiency in the various craniofacial cartilages. Anat Rec 255:452-457
Jee WSS (1988) The skeletal tissues. In: Weiss L (ed.) Cell and tissue biology: a textbook of histology, 6th edn. Urban & Schwarzenberg, Baltimore, pp 211-254
Karaplis AC, Luz A, Lowacki JG, Bronson RT, Tybulewicz VLJ, Kronenberg HM, Mulligan RC (1994) Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev 8:277-289
Kitahara T, Suda N, Kuroda T, Beck F, Hammond VE, Takano Y (2002) Disrupted tooth development in parathyroid hormone-related protein (PTHrP)-gene knockout mice. Bone 30 48-56
Lewinson D, Silbermann M (1992) Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat Rec 233, 504-514
Liu JG, Tabata M, Yamashita K, Matsumura T, Iwamoto M, Kuris K (1998) Developmental role of PTHrP in murine molars. Eur J Oral Sci 106:143-146
Luder HU, Leblond CP, Von Der Mark K (1988) Cellular stages in cartilage formation as revealed by morphometry, radioautography and Type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat 182:197-214
Mekaapiruk K, Suda N, Hammond VE, Beck F, Kuroda T, Takano Y, Terashima T (2002) The influence of parathyroid hormone-related protein (PTHrP) on tooth-germ development and osteoclastogenesis in alveolar bone of PTHrP-knockout and wild-type mice in vivo. Arch Oral Biol, 47:665-672
Nomura S, Wills AJ, Edwards DS, Heath JK, Hogan BLM (1988) Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol 106:441-450
Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broudas AE, Stewart AF (1996) Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev 76:127-173
Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC (1998) Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci U S A 95:11846-11851
Sato M, Yasui N, Nakase T, Kawahata H, Sugimoto M, Hirota S, Kitamura Y, Nomura S, Ochi T (1998) Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res 13:1221-1231
Shibata S, Suda N, Yamazaki K, Kuroda T, Beck F, Senior PV, Hammond VE (2000) Mandibular deformities in parathyroid hormone-related protein (PTHrP) deficient mice: possible involvement of masseter muscle. Anat Embryol (Berl) 202:85-93
Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y (2001) Immunohistochemical localisation of versican, aggrecan and hyaluronan in developing rat mandibular condylar cartilage. J Anat 198:129-135
Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y (2002) In situ hybridization and immunohistochemistry of bone sialoprotein and secreted phosphoprotein 1 (osteopontin) in the developing mouse mandibular condylar cartilage compared with limb bud cartilage. J Anat 200:309-320
Sommer B, Bickel M, Hofstetter W, Wetterwald A (1996) Expression of matrix proteins during the development of mineralized tissues. Bone 19:371-380
Sperber GH (2001) Craniofacial Development. BC Decker, Hamilton,Canada
Suda N, Shibata S, Yamazaki K, Kuroda T, Senior PV, Beck F, Hammond VE (1999) Parathyroid hormone-related protein (PTHrP) regulates proliferation of condylar hypertrophic chondrocytes. J Bone Miner Res 14:1838-1847
Suda N, Baba O, Udagawa N, Terashima T, Takano Y, Kuroda T, Senior PV, Beck F, Hammond VE (2001) Parathyroid hormone-related protein (PTHrP) is required for normal intramembranous bone development. J Bone Miner Res 16:2182-2191
Stutzmann J, Petrovic A (1975) Nature et aptitudes evolutives des cellules du compartiment mitotique des cartilages secondaires de la mandibule et du maxillaire de jeune rat. Experiences de culture cytotypique et d'homotransolantation. Bull Assoc Anat 59:523-534
Tomo S, Ogita M, Tomo I (1997) Development of mandibular cartilages in the rat. Anat Rec 249: 233-239
Tucci J, Hammond V, Senior PV, Gibson A, Beck F (1996) The role of fetal parathyroid hormone-related protein in transplacental calcium transport. J Mol Endocrinol 17:159-164
Vinkka H (1982) Secondary cartilages in the facial skeleton of the rat. Proc Finn Dent Soc [Suppl 78] 7:1-137
Yasui N, Sato M, Ochi T, Kimura T, Kawahata H, Kitamura Y, Nomura S (1997) Three models of ossification during distraction osteogenesis in the rat. J Bone Joint Surg 79: 824-830
Acknowledgements
The monoclonal antibody 12/21/1C6 developed by B. Caterson (Connective Tissue Biology Lab, School of Molecular and Medical Biosciences) were obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, under contact NO1-HD-&-3263 from the NICHD. This work is supported by Grant-in-Aid for Scientific Research (No. 12671762) from Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibata, S., Suda, N., Fukada, K. et al. Mandibular coronoid process in parathyroid hormone-related protein-deficient mice shows ectopic cartilage formation accompanied by abnormal bone modeling. Anat Embryol 207, 35–44 (2003). https://doi.org/10.1007/s00429-003-0325-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-003-0325-6