Abstract
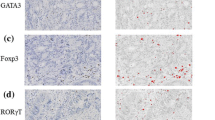
Lymphocyte-rich gastric carcinomas (Ly-rich GCs) are characterized by the formation of lymphoid stroma. Our previous study has shown abundant infiltration of CXCR3+ T cells and their frequent clustering with CXCL9 (monokine induced by interferon-γ)-expressing stromal cells in the lymphoid stroma of Ly-rich GCs. Foxp3+ regulatory T cells (Tregs) suppress immune responses in the peripheral tissues and play a role in the immunosuppression of cancer tissues. The present study was therefore undertaken to evaluate the significance of the balance between CXCR3+ T cells and Tregs in 44 Ly-rich and 37 conventional GCs by morphometrical analyses of immunohistochemistry. Compared with the pronounced infiltration of CXCR3+ T cells in the lymphoid stroma, the numbers of Foxp3+ cells were relatively low in Ly-rich GCs. Therefore, the ratios of CXCR3+/Foxp3+ cells were much higher in Ly-rich GCs than in conventional GCs. This suggests the occurrence of T-helper type 1 (Th1)-shifted immune responses in Ly-rich GCs. On the other hand, conventional GCs were characterized by a paucity of CXCR3+ T cells and a relative abundance of Tregs. Furthermore, the stroma inside the cancer was characterized by even less CXCR3+ cells, suggesting a strongly immunosuppressive microenvironment. Since Tregs are known to express CCR4, we also examined the tissue distribution of cells expressing its ligand CCL22. CCL22 was not detected in conventional GCs and only sparsely detected in dendritic cells but not in cancer cells in Ly-rich GCs. To conclude, Tregs may play a more important role in conventional GCs than in Ly-rich GCs from the viewpoint of immunosuppression.





Similar content being viewed by others
References
Yu P, Fu YX (2006) Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest 86:231–245. doi:10.1038/labinvest.3700389
Naito Y, Saito K, Shiiba K et al (1998) CD8+ T-cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Dave SS, Wright G, Tan B et al (2004) Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 351:2159–2169
Sato E, Olson SH, Ahn J et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543. doi:10.1073/pnas.0509182102
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. doi:10.1126/science.1129139
Sharma P, Shen Y, Wen S et al (2007) CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA 104:3967–3972. doi:10.1073/pnas.0611618104
Watanabe H, Enjoji M, Imai T (1976) Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer 38:232–243
Minamoto T, Mai M, Watanabe K et al (1990) Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer 66:945–952
Tokunaga M, Land CE, Uemura Y et al (1993) Epstein-Barr virus in gastric carcinoma. Am J Pathol 143:1250–1254
Fukayama M, Hino R, Uozaki H (2008) Epstein-Barr virus and gastric carcinoma: virus-host interactions leading to carcinoma. Cancer Sci 99(9):1726–1733. doi:10.1111/j.1349-7006.2008.00888
Yoshie O, Imai T, Nomiyama H (2001) Chemokines in immunity. Adv Immunol 78:57–110
Krieg C, Boyman O (2009) The role of chemokines in cancer immune surveillance by the adaptive immune system. Semin Cancer Biol 19:76–83. doi:10.1016/j.semcancer.2008.10.011
Ohtani H, Jin Z, Takegawa S et al (2009) Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte-rich gastric carcinoma. J Pathol 217:21–31. doi:10.1002/path.2448
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360. doi:10.1146/annurev.immunol.22.012703.104803
Finn OJ (2008) Cancer immunology. N Engl J Med 358(25):2704–2715. doi:10.1056/NEJMra072739
Josefowicz SZ, Rudensky A (2009) Control of regulatory T cell lineage: commitment and maintenance. Immunity 30:616–625. doi:10.1016/j.immuni.2009.04.009
Shevach EM (2009) Mechanisms of FoxP3+ T regulatory cell-mediated suppression. Immunity 30:636–645. doi:10.1016/j.immuni.2009.04.010
Sobin LH, Gospodarowicz MK, Wittekind Ch (eds) (2009) TNM classification of malignant tumours, 7th edn. UK, Wiley-Blackwell, Oxford
Kryczek I, Liu R, Wang G (2009) FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 69:3995–4000. doi:10.1158/0008-5472.CAN-08-3804
Musha H, Ohtani H, Mizoi T et al (2005) Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. Int J Cancer 116:949–956
Lauren P (1965) The two histologic main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma, 13th ed. Kanehara, 2001, Tokyo, Japan (in Jpn).
Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949. doi:10.1038/nm1093
Gao Q, Qiu SJ, Fan J et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593. doi:10.1200/JCO.2006.09.4565
Jordanova ES, Gorter A, Ayachi O et al (2008) Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res 14:2028–2035. doi:10.1158/1078-0432.CCR-07-4554
Mizukami Y, Kono K, Kawaguchi Y et al (2008) Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer 98:148–153. doi:10.1038/sj.bjc.6604|49
Mizukami Y, Kono K, Kawaguchi Y et al (2008) CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer 122:2286–2293. doi:10.1002/ijc.23392
Muthuswamy R, Urban J, Lee JJ et al (2008) Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res 68:5972–5978. doi:10.1158/0008-5472.CAN-07-6818
Wu MS, Huang SP, Chang YT (2002) Tumor necrosis factor-alpha and interleukin-10 promoter polymorphisms in Epstein-Barr virus-associated gastric carcinoma. J Infect Dis 185:106–109
Korn T, Bettelli E, Oukka M et al (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517. doi:10.1146/annurev.immunol.021908.132710
Acknowledgments
We are grateful to Dr. Masaaki Miyazawa (Kinki University School of Medicine) for critical reading of the manuscript, Dr. Takashi Nakayama (Kinki University School of Medicine) for valuable discussion, Dr. Noriko Kimura (Hakodate National Hospital) and Dr. Hiroshi Naganuma (Sendai City Hospital) for supplying surgical materials, Ms. Fumiko Date (Tohoku University Graduate School of Medicine) for clerical assistance, and Ms. Megumi Maeda and Ms. Shoko Kajiwara (National Institute for Materials Science [NIM.S.]–Leica Bioimaging Laboratory) for technical assistance in confocal laser scanning microscopy. The present study was partly supported by the National Hospital Organization Collaborative Clinical Research Grant as well as the “Nanotechnology Network Project” and “HAITEKU” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figures
The number of CXCR3+ cells and that of Foxp3+ cells show no significance changes with stages in both Ly-rich and conventional GCs (JPEG 205 kb)
Rights and permissions
About this article
Cite this article
Ohtani, H., Yoshie, O. Morphometric analysis of the balance between CXCR3+ T cells and FOXP3+ regulatory T cells in lymphocyte-rich and conventional gastric cancers. Virchows Arch 456, 615–623 (2010). https://doi.org/10.1007/s00428-010-0921-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0921-9