Abstract
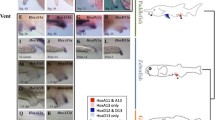
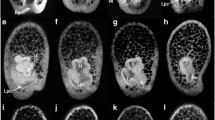
Hox genes are noted for their roles in specifying axial identity in bilateral forms. In the radial echinoderms, the axis whose identity Hox genes might specify remains unclear. From the expression of Hox genes in the development of the sea urchin Holopneustes purpurescens reported here and that reported previously, we clarify the axis that might be specified by Hox genes in echinoderms. The expression of HpHox11/13 here is described at three developmental stages. The expression is around the rim of the blastopore in gastrulae, in the archenteron wall and adjacent mesoderm in early vestibula larvae, and in a patch of mesoderm close to the archenteron wall in later vestibula larvae. The retained expression of HpHox11/13 in the patch of mesoderm in the later vestibula larvae is, we suggest, indicative of a posterior or an aboral growth zone. The expression of HpHox3 at the echinoid-rudiment stage, in contrast, is in oral mesoderm beneath the epineural folds, concentrated in sites where the first three adult spines form. With the expression of HpHox5 and HpHox11/13 reported previously, the expressions here support the role of Hox genes in specifying oral–aboral identity in echinoderms. How such specification and a posterior growth zone add support to a concept of the structural homology between echinoderms and chordates is discussed.





Similar content being viewed by others
References
Arenas-Mena C, Martinez P, Cameron RA, Davidson EH (1998) Expression of the Hox gene complex in the indirect development of a sea urchin. Proc Natl Acad Sci U S A 95:13062–13067
Arenas-Mena C, Cameron AR, Davidson EH (2000) Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development 127:4631–4643
Cameron RA, Rowen L, Nesbitt R, Bloom S, Rast JP, Berney K, Arenas-Mena C, Martinez P, Lucas S, Richardson PM et al (2006) Unusual gene order and organization of the sea urchin Hox cluster. J Exp Zool (Mol Dev Evol) 306B:45–58
Cisternas P, Byrne M (2009) Expression of Hox4 during development of the pentamerous juvenile sea star, Parvulastra exigua. Dev Genes Evol 219:613–618
de Rosa R, Prud'homme B, Balavoine G (2005) caudal and even-skipped in the annelid Platynereis dumerilii and the ancestry of posterior growth. Evol Dev 7:574–587
Duboc V, Lepage T (2008) A conserved role for the Nodal signaling pathway in the establishment of dorso-ventral and left–right axes in deuterostomes. J Exp Zool (Mol Dev Evol) 310B:41–53
Duboc V, Röttinger E, Besnardeau L, Lepage T (2004) Nodal and BMP2/4 signaling organizes the oral–aboral axis of the sea urchin embryo. Dev Cell 6:397–410
Duboc V, Röttinger E, Lapraz F, Besnardeau L, Lepage T (2005) Left–right asymmetry in the sea urchin embryo is regulated by Nodal signaling on the right side. Dev Cell 9:147–158
Ferrier DEK, Holland PWH (2002) Ciona intestinalis ParaHox genes: evolution of Hox/ParaHox cluster integrity, developmental mode, and temporal colinearity. Mol Phylogenet Evol 24:412–417
Garcia-Fernàndez J (2005) Hox, ParaHox, ProtoHox: facts and guesses. Heredity 94:145–152
Hara Y, Yamaguchi M, Akasaka K, Nakano H, Nonaka M, Amemiya S (2006) Expression patterns of Hox genes in larvae of the sea lily Metacrinus rotundus. Dev Genes Evol 216:797–809
Heinzeller T, Welsch U (1999) The complex of notochord/neural plate in chordates and the complex of hydrocoel/ectoneural cord in echinoderms — analogous or homologous? In: Candia Carnevali MD, Bonasoro F (eds) Echinoderm research 1998. Balkema, Rotterdam, pp 285–290
Hotchkiss FHC (2012) Growth zones and extraxial–axial skeletal homologies in Asteroidea (Echinodermata). Proc Biol Soc Wash 125:106–121
Kmita M, Duboule D (2003) Organizing axes in time and space; 25 years of colinear tinkering. Science 301:331–333
Lewis EB (1978) A gene complex controlling segmentation in Drosophila. Nature 276:565–570
Long S, Martinez P, Chen W-C, Thorndyke M, Byrne M (2003) Evolution of echinoderms may not have required modification of the ancestral deuterostome HOX gene cluster: first report of PG4 and PG5 Hox orthologues in echinoderms. Dev Genes Evol 213:573–576
Mallo M, Wellik DM, Deschamps J (2010) Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344:7–15
McGinnis W, Krumlauf R (1992) Homeobox genes and axial patterning. Cell 68:283–302
Minsuk SB, Turner FR, Andrews ME, Raff RA (2009) Axial patterning of the pentaradial adult echinoderm body plan. Dev Genes Evol 219:89–101
Mooi R, David B, Marchand D (1994) Echinoderm skeletal homologies: classical morphology meets modern phylogenetics. In: David B, Guille A, Féral JP, Roux M (eds) Echinoderms through time. Balkema, Rotterdam, pp 87–95
Mooi R, David B, Wray GA (2005) Arrays in rays: terminal addition in echinoderms and its correlation with gene expression. Evol Dev 7:542–555
Morris VB (1995) Apluteal development of the sea urchin Holopneustes purpurescens Agassiz (Echinodermata: Echinoidea: Euechinoidea). Zool J Linnean Soc Lond 114:349–364
Morris VB (2007) Origins of radial symmetry identified in an echinoderm during adult development and the inferred axes of ancestral bilateral symmetry. Proc R Soc B 274:1511–1516
Morris VB (2009) On the sites of secondary podia formation in a juvenile echinoid: growth of the body types in echinoderms. Dev Genes Evol 219:597–608
Morris VB (2011) Coelomogenesis during the abbreviated development of the echinoid Heliocidaris erythrogramma and the developmental origin of the echinoderm pentameral body plan. Evol Dev 13:370–381
Morris VB (2012) Early development of coelomic structures in an echinoderm larva and a similarity with coelomic structures in a chordate embryo. Dev Genes Evol 222:313–323
Morris VB, Byrne M (2005) Involvement of two Hox genes and Otx in echinoderm body-plan morphogenesis in the sea urchin Holopneustes purpurescens. J Exp Zool (Mol Dev Evol) 304B:456–467
Morris VB, Brammall J, Byrne M, Frommer M (2002) cDNA Hox sequences 3′ of the homeobox isolated from the sea urchin Holopneustes purpurescens are definitive for sea urchin Hox orthologues. DNA Sequence 13:185–193
Morris VB, Zhoa J-T, Shearman DCA, Byrne M, Frommer M (2004) Expression of an Otx gene in the adult rudiment and the developing central nervous system in the vestibula larva of the sea urchin Holopneustes purpurescens. Int J Dev Biol 48:17–22
Peter IS, Davidson EH (2011) A gene regulatory network controlling the embryonic specification of endoderm. Nature 474:635–639
Peterson KJ, Arenas-Mena C, Davidson EH (2000) The A/P axis in echinoderm ontogeny and evolution: evidence from fossils and molecules. Evol Dev 2:93–101
Schier AF (2003) Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 19:589–621
Smith MM, Smith LC, Cameron RA, Urry LA (2008a) The larval stages of the sea urchin, Strongylocentrotus purpuratus. J Morphol 269:713–733
Smith MS, Turner FR, Raff RA (2008b) Nodal expression and heterochrony in the evolution of dorsal–ventral and left–right axes formation in the direct-developing sea urchin Heliocidaris erythrogramma. J Exp Zool (Mol Dev Evol) 310B:609–622
Turner RL (1998) The metameric echinoderm. In: Mooi R, Telford M (eds) Echinoderms: San Francisco. Balkema, Rotterdam, p 89
von Ubisch L (1913) Die Entwicklung von Strongylocentrotus lividus (Echinus microtuberculatus, Arbacia pustulosa.). Zeit f wiss Zool 106:409–448
Acknowledgments
We acknowledge the facilities and the scientific and technical assistance from staff of the Australian Centre for Microscopy and Microanalysis at the University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Hiroki Nishida
Rights and permissions
About this article
Cite this article
Morris, V.B., Byrne, M. Oral–aboral identity displayed in the expression of HpHox3 and HpHox11/13 in the adult rudiment of the sea urchin Holopneustes purpurescens . Dev Genes Evol 224, 1–11 (2014). https://doi.org/10.1007/s00427-013-0457-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-013-0457-5