Abstract
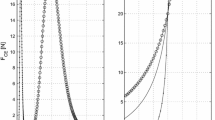
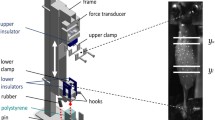
High-frequency vibrations e.g., induced by legs impacting with the ground during terrestrial locomotion can provoke damage within tendons even leading to ruptures. So far, macroscopic Hill-type muscle models do not account for the observed high-frequency damping at low-amplitudes. Therefore, former studies proposed that protective damping might be explained by modelling the contractile machinery of the muscles in more detail, i.e., taking the microscopic processes of the actin–myosin coupling into account. In contrast, this study formulates an alternative hypothesis: low but significant damping of the passive material in series to the contractile machinery—e.g., tendons, aponeuroses, titin—may well suffice to damp these hazardous vibrations. Thereto, we measured the contraction dynamics of a piglet muscle–tendon complex (MTC) in three contraction modes at varying loads and muscle–tendon lengths. We simulated all three respective load situations on a computer: a Hill-type muscle model including a contractile element (CE) and each an elastic element in parallel (PEE) and in series (SEE) to the CE pulled on a loading mass. By comparing the model to the measured output of the MTC, we extracted a consistent set of muscle parameters. We varied the model by introducing either linear damping in parallel or in series to the CE leading to accordant re-formulations of the contraction dynamics of the CE. The comparison of the three cases (no additional damping, parallel damping, serial damping) revealed that serial damping at a physiological magnitude suffices to explain damping of high-frequency vibrations of low amplitudes. The simulation demonstrates that any undamped serial structure within the MTC enforces SEE-load eigenoscillations. Consequently, damping must be spread all over the MTC, i.e., rather has to be de-localised than localised within just the active muscle material. Additionally, due to suppressed eigenoscillations Hill-type muscle models taking into account serial damping are numerically more efficient when used in macroscopic biomechanical neuro-musculo-skeletal models.
Similar content being viewed by others
Abbreviations
- MTC:
-
Muscle–tendon complex
- CE:
-
Contractile element
- PE:
-
Parallel element
- PEE:
-
Parallel elastic element
- SE:
-
Serial element
- SEE:
-
Serial elastic element
- SOL:
-
M. soleus
- FDS:
-
M. flexor digitorum superficialis
- GM:
-
M. gastrocnemius medialis
- GL:
-
M. gastrocnemius lateralis
- q :
-
normalised muscle activation
- q0:
-
minimum value of q
- \(\dot{q}\) :
-
time derivative of q
- τ q :
-
time constant of rising activation
- β q :
-
ratio between τ q and time constant of falling activation
- STIM:
-
Muscle stimulation
- l CE :
-
length of CE
- \(\dot{l}_{{\rm CE}} = v_{{\rm CE}}\) :
-
contraction velocity of CE
- l 0 :
-
mean anatomical length of MTC
- l m :
-
length of model MTC
- l m,0 :
-
typical length of model MTC
- \(\dot{l}_{{\rm m}}\) :
-
velocity of model MTC
- F m :
-
force of model MTC
- F SEE :
-
force of SEE
- F PEE :
-
force of PEE
- F CE :
-
force of CE
- l SE :
-
length of SE
- l SEE,0 :
-
rest length of SEE
- l SEE,nll :
-
length of SEE at non-linear-linear transition in F SEE(l SE)
- Δ U SEE,nll :
-
relative stretch at non-linear-linear transition in F SEE(l SE)
- Δ F SEE,0 :
-
force at non-linear-linear transition in F SEE(l SE)
- Δ U SEE,l :
-
relative stretch in linear part for force increase ΔF SEE,0
- K SEE,l :
-
stiffness of the linear part of F SEE(l SE)
- ν SEE :
-
exponent of F SEE(l SE) in the non-linear part
- K SEE,nl :
-
factor of non-linearity in F SEE(l SE)
- F isom :
-
normalised isometric force–length relation of CE
- l CE,opt :
-
optimal fibre length
- ν CE,limb :
-
exponent of F isom(l CE) on either ascending or descending limb
- ΔW limb :
-
width of F isom(l CE) on either ascending or descending limb
- F max :
-
maximum isometric force
- A rel :
-
coordinate of pole in \(\dot{l}_{{\rm CE}}(F_{{\rm CE}})\) normalised to current isometric force F max q F isom(l CE)
- A rel,0 :
-
maximum value of A rel
- B rel :
-
coordinate of pole in \(F_{{\rm CE}}(\dot{l}_{CE})\) normalised to l CE,opt
- B rel,0 :
-
maximum value of B rel
- v max :
-
concentric contraction velocity at F CE = 0
- v max,0 :
-
maximum concentric contraction velocity
- \(L_{A_{{\rm rel}}}\) :
-
length dependency of A rel
- \(L_{B_{{\rm rel}}}\) :
-
length dependency of B rel
- \(Q_{A_{{\rm rel}}}\) :
-
activation dependency of A rel
- \(Q_{B_{{\rm rel}}}\) :
-
activation dependency of B rel
- l PEE,0 :
-
rest length of PEE
- ν PEE :
-
exponent of F PEE(l CE )
- K PEE :
-
factor of non-linearity in F PEE(l CE)
- \({\mathcal{F}}_{{\rm PEE}}\) :
-
force of PEE if l CE is stretched to ΔW limb=des
- \({\mathcal{L}}_{{\rm PEE},0}\) :
-
rest length of PEE normalised to l CE,opt
- F EPS :
-
numerical limit for defining zero F isom(l CE)
- d V d F con :
-
inclination of linear concentric continuation of \(\dot{l}_{{\rm CE}}(F_{{\rm CE}})\) for F CE < 0
- S ecc :
-
step in inclination of \(F_{{\rm CE}}(\dot{l}_{{\rm CE}} = 0)\) between eccentric and concentric force–velocity relation(s)
- \({\mathcal{F}}_{{\rm ecc}}\) :
-
coordinate of pole in \(\dot{l}_{{\rm CE}}(F_{{\rm CE}})\) normalised to F max q F isom(l CE) for \(\dot{l}_{{\rm CE}} > 0\)
- F trans :
-
force where linear continuation of eccentric \(\dot{l}_{{\rm CE}}(F_{{\rm CE}})\) relation starts
- v trans :
-
velocity where linear continuation of eccentric \(\dot{l}_{{\rm CE}}(F_{{\rm CE}})\) relation starts
- d V d F ecc :
-
inclination of linear eccentric continuation of \(\dot{l}_{{\rm CE}}(F_{{\rm CE}})\) for F CE > F trans
- l PE :
-
length of PEE ( = l CE)
- d PE :
-
(constant) damping coefficient of PE
- d SE :
-
damping coefficient of SE
- d SE,max :
-
maximum value in d SE(l CE, q)
- R SE :
-
minimum value of d SE normalised to d SE,max
- D SE :
-
dimensionless factor to scale d SE,max
- \(\dot{l}_{{\rm SE}} = v_{{\rm SE}}\) :
-
contraction velocity of SE
- t :
-
time
- g :
-
vector of gravitational acceleration
- d ext :
-
modelled external damping
References
Alexander RMcN (1988) Elastic mechanisms in animal movement. Cambrigde University Press, Cambridge
Alexander RMcN (2001) Damper for bad vibrations. Nature 414(6866):855–857
Biewener AA (1990) Biomechanics of mammalian terrestrial locomotion. Science 250(4984):1097–1103
Blickhan R (1989) The spring-mass model for running and hopping. J Biomech 22(11/12):1217–1227
Carrion-Vazquez M, Oberhauser AF, Fowler SB, Marszalek PE, Broedel SE, Clarke J, Fernandez JM (1999) Mechanical and chemical unfolding of a single protein: a comparison. Proc Natl Acad Sci USA 96(7):3694–3699
Cavagna GA (1970) Elastic bounce of the body. J Appl Physiol 29(3):279–282
Chow JW, Darling WG (1999) The maximum shortening velocity of muscle should be scaled with activation. J Appl Physiol 86(3):1025–1031
Currey JD (2002) Bones: structure and mechanics. Princeton University Press, Princeton, NJ
Davy DT, Audu ML (1987) A dynamic optimization technique for predicting muscle forces in the swing phase of gait. J Biomech 20(2):187–201
Denoth J (1985) The dynamic behaviour of a three link model of the human body during impact with the ground. In: Winter DA, Norman RW, Wells RP, Hayes KC, Patla AE (eds) Biomechanics 9-A, vol 5B of International Series on Biomechanics. Human Kinetics Publishers, Champaign, pp 102–106
Denoth J, Stüssi E, Csucs G, Danuser G (2002) Single muscle fiber contraction is dictated by inter-sarcomere dynamics. J Theor Biol 216(1):101–122
Epstein M, Herzog W (2003) Aspects of skeletal muscle modelling. Philos Trans R Soc Lond B 358(1437):1445–1452
Ettema GJ, Meijer K (2000) Muscle contraction history: modified Hill versus an exponential decay model. Biol Cybern 83(6):491–500
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibers. J Physiol 184:170–192
Granzier HL, Labeit S (2006) The giant muscle protein titin is an adjustable molecular spring. Exerc Sport Sci Rev 34(2):50–53
Gruber K, Ruder H, Denoth J, Schneider K (1998) A comparative study of impact dynamics: wobbling mass model versus rigid body models. J Biomech 31(5):439–444
Günther M, Ruder H (2003) Synthesis of two-dimensional human walking: a test of the λ-model. Biol Cybern 89(2):89–106
Günther M, Sholukha VA, Keßler D, Wank V, Blickhan R (2003) Dealing with skin motion and wobbling masses in inverse dynamics. J Mech Med Biol 3(3/4):309–335
Hatze H (1977) A myocybernetic control model of skeletal muscle. Biol Cybern 25:103–119
Hatze H (1981) Myocybernetic control models of skeletal muscle—characteristics and applications. University of South Africa Press, Pretoria
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B 126:136–195
Julian FJ (1971) The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol 218:117–145
Katz B (1939) The relation between force and speed in muscular contraction. J Physiol 96:45–64
Ker RF (1981) Dynamic tensile properties of the plantaris tendon of sheep (Ovis aries). J Exp Biol 93:283–302
Ker RF, Wang XT, Pike AV (2000) Fatigue quality of mammalian tendons. J Exp Biol 203(Pt 8):1317–1327
Ker RF, Zioupos P (1997) Creep and fatigue damage of mammalian tendon and bone. Comments Theor Biol 4(2-3):151–181
Kistemaker DA, van Soest AJ, Bobbert MF (2006) Is equilibrium point control feasible for fast goal-directed single-joint movements? J Neurophysiol 95(5):2898–2912
Krause PC, Choi JS, McMahon TA (1995) The force-velocity curve in passive whole muscle is asymmetric about zero velocity. J Biomech 28(9):1035–1043
Krieg M (1992) Simulation und Steuerung biomechanischer Mehrkörpersysteme. Master’s thesis. Eberhard-Karls-Universität, Tübingen, Germany
Lan G, Sun SX (2005) Dynamics of myosin-driven skeletal muscle contraction: I. Steady-state force generation. Biophys J 88(6):4107–4117
Lombardi V, Piazzesi G, Ferenczi MA, Thirlwell H, Dobbie I, Irving M (1995) Elastic distortion of myosin heads and repriming of the working stroke in muscle. Nature 374(6522):553–555
Lombardi V, Piazzesi G, Reconditi M, Linari M, Lucii L, Stewart A, Sun YB, Boesecke P, Narayanan T, Irving T, Irving M (2004) X-ray diffraction studies of the contractile mechanism in single muscle fibres. Philos Trans R Soc Lond B 359(1452):1883–1893
Marszalek PE, Lu H, Li H, Carrion-Vazquez M, Oberhauser AF, Schulten K, Fernandez JM (1999) Mechanical unfolding intermediates in titin modules. Nature 402(6757):100–103
McMahon TA, Cheng GC (1990) The mechanics of running: how does stiffness couple with speed? J Biomech 23(Suppl. 1):65–78
Meijer K, Grootenboer HJ, Koopman HF, van der Linden BJ, Huijing PA (1998) A Hill type model of rat medial gastrocnemius muscle that accounts for shortening history effects. J Biomech 31(6):555–563
Minajeva A, Kulke M, Fernandez JM, Linke WA (2001) Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils. Biophys J 80(3):1442–1451
Pain MTG, Challis JH (2006) The influence of soft tissue movement on ground reaction forces, joint torques and reaction forces in drop landings. J Biomech 39(1):119–124
Petrofsky JS, Phillips CA (1981) The influence of temperature, initial length and electrical activity on force-velocity relationship of the medial gastrocnemius muscle of the cat. J Biomech 14(5):297–306
Piazzesi G, Lombardi V (1995) A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys J 68(5):1966–1979
Piazzesi G, Lombardi V (1996) Simulation of the rapid regeneration of the actin-myosin working stroke with a tight coupling model of muscle contraction. J Muscle Res Cell Motil 17(1):45–53
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (1994) Numerical recipes in C—the art of scientific computing, 2nd edn. Cambridge University Press, Cambridge
Proske U, Morgan DL (1987) Tendon stiffness: methods of measurement and significance for the control of movement. A review. J Biomech 20(1):75–82
Reconditi M, Linari M, Lucii L, Stewart A, Sun YB, Boesecke P, Narayanan T, Fischetti RF, Irving T, Piazzesi G, Irving M, Lombardi V (2004) The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature 428(6982):578–581
Reinsch CH (1967) Smoothing by spline functions. Numerische Mathematik 10(3):177–183
Riemersma DJ, Schamhardt HC (1985) In vitro mechanical properties of equine tendons in relation to cross-sectional area and collagen content. Res Vet Sci 39(3):263–270
Rode C, Siebert T, Herzog W, Blickhan R (2006) The effects of parallel and series elastic components on estimated active muscle force (submitted to the J Biomech)
Schmalz T (1993a) Biomechanische Modellierung menschlicher Bewegung, vol 26 of Wissenschaftliche Schriftenreihe des Deutschen Sportbundes. Karl Hofmann, Schorndorf, Germany
Schmalz T (1993b) Die Nutzung biomechanischer Modelle zur Bestimmung rheologischer Eigenschaften des Muskel-Sehnen-Komplexes. In: Gutewort W, Schmalz T, Weiß T (eds), Symposium Oberhof: Aktuelle Hauptforschungsrichtungen der Biomechanik sportlicher Bewegungen, vol 55, Sankt Augustin, Germany, 1993. Deutsche Vereinigung für Sportwissenschaft (dvs), Academia, pp 102–108
Shadwick RE (1990) Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol 68(3):1033–1040
Shampine LF, Gordon MK (1975) Computer solution of ordinary differential equations: the initial value problem. W.H. Freeman & Co., San Francisco
Siebert T, Wagner H, Blickhan R (2003) Not all oscillations are rubbish: forward simulation of quick-release experiments. J Mech Med Biol 3(1):107–122
Stern JT (1974) Computer modeling of gross muscle dynamics. J Biomech 7:411–428
Telley IA, Denoth J, Ranatunga KW (2003) Inter-sarcomere dynamics in muscle fibres. A neglected subject? Adv Exp Med Biol 538:481–500
Tskhovrebova L, Trinick J (2002) Role of titin in vertebrate striated muscle. Philos Trans R Soc Lond B 357(1418):199–206
van Ingen Schenau GJ (1984) An alternative view to the concept of utilization of elastic energy. Hum Mov Sci 3:301–336
van Leeuwen JL (1992) Muscle function in locomotion. In: Alexander RMcN (ed) Advances in comparative and environmental physiology, vol 11, chap 7. Springer, Berlin, pp 191–250
van Soest AJ (1992) Jumping from structure to control: a simulation study of explosive movements. PhD thesis, Vrije Universiteit, Amsterdam
van Soest AJ, Bobbert MF (1993) The contribution of muscle properties in the control of explosive movements. Biol Cybern 69(3):195–204
Wakeling JM, Nigg BM (2001) Soft-tissue vibrations in the quadriceps measured with skin mounted transducers. J Biomech 34(4):539–543
Wank V (2000) Aufbau und Anwendung von Muskel-Skelett-Modellen. Habilitationsschrift der Friedrich-Schiller-Universität Jena
Wank V, Bauer R, Walter B, Kluge H, Fischer MS, Blickhan R, Zwiener U (2000) Accelerated contractile function and improved fatigue resistance of calf muscles in newborn piglets with IUGR. Am J Physiol Regul Integr Comp Physiol 278(2):R304–R310
Wank V, Fischer MS, Walter B, Bauer R (2006) Muscle growth and fiber type composition in hind limb muscles during postnatal development in pigs. Cells Tissues Organs 182(3-4):171–181
Wilson AM, Goodship AE (1994) Exercise-induced hyperthermia as a possible mechanism for tendon degeneration. J Biomech 27(7):899–905
Wilson AM, McGuigan MP, Su A, van den Bogert AJ (2001) Horses damp the spring in their step. Nature 414(6866):895–899
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. In: Bourne JR (ed) CRC critical reviews in biomedical engineering, vol 17. CRC Press, Boca Raton, pp 359–411
Zioupos P, Currey JD, Casinos A (2001) Tensile fatigue in bone: are cycles-, or time to failure, or both, important? J Theor Biol 210(3):389–399
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Günther, M., Schmitt, S. & Wank, V. High-frequency oscillations as a consequence of neglected serial damping in Hill-type muscle models. Biol Cybern 97, 63–79 (2007). https://doi.org/10.1007/s00422-007-0160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-007-0160-6