Abstract
Background
The purpose of this study is to evaluate the role, the safety and the effectiveness of intravitreal bevacizumab (IVB) injections as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy (PDR).
Design
Case-Control Study
Methods
Randomized controlled trial performed on 72 eyes of 68 patients affected by vitreous haemorrhage (VH) and tractional retinal detachment (TRD), which occurred as a consequence of active proliferative diabetic retinopathy (PDR). We randomly assigned eligible patients in a 1: 1: 1 ratio to receive a sham injection or an intravitreal injection of 1.25 mg of bevacizumab, either 7 or 20 days before the vitrectomy. In order to obtain three homogeneous groups of surgical complexity, we assigned to the following preoperative parameters a score from 0 to 3: a) vitreous haemorrhage, b) prior retinal laser-photocoagulation, c) morphological types of retinal detachment such as focal, hammock, central diffuse, table-top. Complete ophthalmic examinations and color fundus photography were performed at baseline and 1, 6, 12, and 24 weeks after the surgery.
Main outcome measures
Intraoperative management, safety, efficacy of IVB at different time injection as an adjunct to vitrectomy in the management of severe PDR
Results
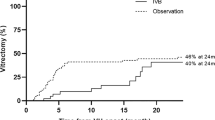
Group A (sham injection): intraoperative bleeding occurred in 19 cases (79.1%), the use of endodiathermy was necessary in 13 patients (54.1%), relaxing retinotomy was performed on one patient (4.1%), and in four cases (16.6%) iatrogenic retinal breaks occurred. The surgical mean time was 84 minutes (SD 12 minutes). Group B (bevacizumab administered 7 days before vitrectomy): intraoperative bleeding occurred in two cases (8.3%) and the use of endodiathermy was necessary in two patients (8.3%). No iatrogenic breaks occurred during the surgery. The surgical mean time was 65 minutes (SD 18 minutes). Group C (bevacizumab administered 20 days before vitrectomy): intraoperative bleeding occurred in three cases (12.5%), the use of endodiathermy was necessary in three patients (1.5%), and an iatrogenic break occurred in one patient (4.1%) while the delamination of fibrovascular tissue was being performed. The surgical mean time was 69 minutes (SD 21 minutes). The average difference in the surgical time was statistically significant between group A and group B (p = 0.025), and between group A and group C (p = 0.031). At the end of the surgery, the retina was completely attached in all eyes. At the 6-month follow-up, we observed the development of tractional retinal detachment (TRD) in one out of 24 patients from group C (4%).
Conclusions
A preoperative intravitreal injection of bevacizumab may represent a new strategy for the surgical treatment of severe PDR by reducing retinal and iris neovascularization: this would make surgery much easier and safer, thus improving the anatomical and functional prognosis. According to our study, the best surgical results are achieved performing the IVB 7 days preoperatively.
Similar content being viewed by others
References
Avanta A, Algvere PV, Berglin L, Seregard S (1996) Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37:1929–1934
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT (1997) Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81:154–162
Otani A, Taxagi H, Oh H, Koyama S, Ogura Y, Matumura M, Honda Y (2002) Vascular endothelial growth factor family and receptor expression in human choroidal neovascular membranes. Microvasc Res 64:162–169
Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118:445–450
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP (1996) Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in non humane primate. Arch Ophthalmol 114:964–970
Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adamis AP (1998) Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 105:232–237
Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N (2000) Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. Am J Pathol 156:1337–1334
Adamis AP, Shima DT (2005) The role of vascular endothelial growth factor in ocular health and disease. Retina 25:111–118
Ferrara N (2002) Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 29:10–14
Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF- receptor chimeric proteins. Proc Natl Acad Sci USA 92:10457–10461
Eyetech Study Group (2003) Antivascular endothelial growth factor therapy for subfoveal choroideal neovascularization secondary to age-related macular degeneration. Phase II study results. Am J Ophthalmol 110:979–986
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group (2004) Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351:2805–2816
Ng EW, Svima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP (2006) Pegaptanib, a targeted anti-VEGF optamer for ocular vascular disease. Nat Rev Drug Discov 5:123–132
VEGF inhibition study in ocular neovascularization (V.I.S.I.O.N.) clinical trial group (2006) Pegaptanib sodium for neovascular age-related macular degeneration. two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophtalmology 113:992–1001
VEGF inhibition study in ocular neovascularization (V.I.S.I.O.N.) clinical trial group (2006) Two-year efficacy results of the two randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 113:1508–1525
Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA, Reimann JD, Greene WL, Shams N (2005) Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112:1048–1053
Rosenfeld PJ, Hein JS, Hantsbarger G, Shams N (2006) Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology 113:631–632
Ferrara N, D’amico L, Shams N, Lowman M, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870
Heier JS, Antoszyk AN, Pavak PR, Leff SR, Rosenfeld PJ, Ciulla TA, Dreyer RF, Gentile RC, Sy JP, Hantsbarger G, Shams N (2006) Ranibizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 113:633–642
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 335:1419–1431
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 335:1432–1444
Ferrara N, Millan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature 3:391–400
Marshall J (2005) The role of bevacizumab as first-line therapy for colon cancer. Semin Oncol 32:543–547
Beer PM, Wong SJ, Hammad AM, Falk NS, O’Malley MR, Khan S (2006) Vitreous level of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina 26:871–876
Shahar JS, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I, Loewenstein A (2006) Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab. Retina 26:262–269
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS (2005) Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112:1035–1047
Laud K, Spaide RF, Freund KB, Slakter J, Klancnik JM Jr (2006) Treatment of choroidal neovascularization in pathologic myopia with intravitreal bevacizumab. Retina 26:960–963
Tewari A, Dhalla MS, Apte RS (2006) Intravitreal bevacizumab for treatment of choroidal neovascularization in pathologic myopia. Retina 26:1093–1094
Nguyen SD, Sham S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA (2005) Bevacizumab suppresses choroidal neovascularization caused by pathological myopia. Br J Ophthalmol 89:1368–1370
Spaide RF, Fisher YL (2006) Intravitreal bevacizumab (Avastin) treatment of proliferative retinopathy complicated by vitreous hemorrhage. Retina 26:275–278
Jorge R, Costa RA, Calioli D, Cintra LP, Scott IU (2006) Intravitreal bevacizumab (avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina 26:1006–1013
Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, Berrocal MH, Solis-Vivanco A, Farah ME, Pan-American Collaborative Retina Study Group (2007) Primary intravitreal bevacizumab (avastin) for diabetic macular oedema. results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 114:743–750
di Lauro R, di Lauro R, de Ruggiero P, di Lauro MT, D’Aloia A (2007) Bevacizumab (Avastin) preoperatorio nel trattamento chirurgico della retinopatia diabetica proliferante. Notiziario Soc Oftalmol It 38:39–45
Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund KB, Cooney M, Fine HF (2006) Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina 26:279–284
Pai SA, Shetty R, Vijayan PB, Venkatasubramaniam G, Yadav NK, Shetty BK, Babu RB, Narayana KM (2007) Clinical, anatomic and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol 143:601–606
Rabena MD, Pieramici DJ, Castellarin AA, Nasir MA, Avery RL (2007) Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 27:419–425
Davidorf FM, Mouser JG, Derick RJ (2006) Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina 26:354–356
Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf-Schnurrbursch U, Wolf S, Sarra GM (2006) Bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol 142:1054–1056
Costagliola C, Cipollone U, Rinaldi M, della Corte M, Semeraro F, Romano MR (2008) Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma: a survey on 23 cases throughout 12-month follow-up. Br J Clin Pharmacol 66:667–673
Mason JO, Albert MA Jr, Vail R (2006) Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina 26:356–360
Quiroz-Mercado H, Ustariz-Gonzales O, Martinez-Castellanos MA, Covarrubias P, Dominguez F, Sanchez-Huerta V (2007) Our experience after 1765 intravitreal injections of bevacizumab: the importance of being part of a developing story. Sem Ophthalmol 22:109–125
Travassos A, Teixeira S, Ferreira P, Regadas I, Travassos AS, Esperancinha FE, Prieto I, Pires G, van Velze R, Valido A, Machado Mdo C (2007) Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers 38:233–237
Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B, Essex R, McCombe M, Qureshi S, Campbell WG (2008) Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin Experiment Ophthalmol 36:449–454
Romano MR, Gibran SK, Marticorena J, Wong D, Heimann H (2008) Can a preoperative bevacizumab injection prevent recurrent postvitrectomy diabetic vitreous haemorrhage? Eye 23:1698–1701
Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G (2008) Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 246:837–842
Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A (2006) Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 113:1695
Krohne TU, Eter N, Holz FG, Meyer CH (2008) Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol 146:508–512
Warner T (1999) Relationships between the endothelin and nitric oxide pathways. Clin Exp Pharmacol Physiol 26:247–252
Rosenfeld PJ, Moshfeghi AA, Puliafito CA (2005) Optical coherence tomography findings after intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 36:331–335
Avery RL, Pieramici DJ, Rabena MD, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A (2006) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113:363–372
Spaide RF, Laud K, Fine MF, Klancnik JM Jr, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL, Cooney MJ (2006) Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 26:383–390
Chen E, Park CH (2006) Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 26:699–700
Arevalo JF, Maia M, Flynn HW Jr, Saravia M, Avery RL, Wu L, Eid Farah M, Pieramici DJ, Berrocal MH, Sanchez JG (2008) Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 92:213–216
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trial registration number: NCT01025934.
No research funding.
No proprietary interests.
Rights and permissions
About this article
Cite this article
di Lauro, R., De Ruggiero, P., di Lauro, R. et al. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 248, 785–791 (2010). https://doi.org/10.1007/s00417-010-1303-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1303-3