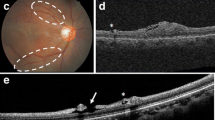
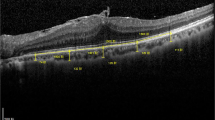
Abstract
Purpose
To develop a reproducible surgical technique for the induction of choroidal neovascularization (CNV) in the subretinal space of porcine eyes and to analyse the resulting CNV clinically and histologically.
Methods
Two different modifications of a surgical technique previously described were compared with the original method. In ten porcine eyes retinal pigment epithelial (RPE) cells were removed using a silicone tipped cannula, in ten porcine eyes Bruch’s membrane was perforated once with a retinal perforator without prior RPE removal and in ten eyes RPE removal was followed by a single perforation of Bruch’s membrane. Fifteen of the eyes, five from each group, were enucleated 30 minutes after surgery, while the remaining eyes were enucleated after 14 days. Prior to enucleation, at day 14, fundus photographs and fluorescein angiograms were obtained. Eyes were examined by light microscopy and by immunohistochemical staining. In addition to these 30 eyes, two eyes underwent surgery with the purpose of subsequent scanning electron microscopic (SEM) examination.
Results
In eyes enucleated immediately after surgery neuroretinas overlying the induced lesions were intact without apparent atrophy of cells regardless of the surgical technique applied. The process of RPE removal was found to induce breaks in Bruch’s membrane and both the size and the number of breaks varied between eyes. CNV membranes were identified in 15 of 15 eyes enucleated after 14 days. CNV membranes induced by perforation of Bruch’s membrane without prior RPE removal were significantly thicker than membranes from eyes undergoing both RPE removal and Bruch’s perforation (p = 0.03) and also thicker than membranes from eyes with only RPE-removal (p < 0.01). CNV membranes from eyes with perforation of Bruch’s membrane without prior RPE removal had a higher cellular content and were more richly vascularized and also exhibited the highest propensity to leak in fluorescense angiograms.
Conclusion
All three surgical techniques were capable of inducing CNV, but the one applying perforation of Bruch’s membrane without RPE removal was easier to reproduce and involved fewer variables than the techniques utilizing RPE removal. The presence of RPE cells seems to affect both the morphology and cellular composition of induced CNV.






Similar content being viewed by others
References
Blaauwgeers HG, Holtkamp GM, Rutten H et al (1999) Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol 155:421–428
Cashman SM, Bowman L, Christofferson J, Kumar-Singh R (2006) Inhibition of choroidal neovascularization by adenovirus-mediated delivery of short hairpin RNAs targeting VEGF as a potential therapy for AMD. Invest Ophthalmol Vis Sci 47:3496–3504
Chang TS, Freund KB, de la Cruz Z, Yannuzzi LA, Green WR (1994) Clinicopathologic correlation of choroidal neovascularization demonstrated by indocyanine green angiography in a patient with retention of good vision for almost four years. Retina 14:114–124
Chin HS, Park TS, Moon YS, Oh JH (2005) Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 25:556–560
Cui JZ, Kimura H, Spee C, Thumann G, Hinton DR, Ryan SJ (2000) Natural history of choroidal neovascularization induced by vascular endothelial growth factor in the primate. Graefes Arch Clin Exp Ophthalmol 238:326–333
Del Priore LV, Hornbeck R, Kaplan HJ et al (1995) Debridement of the pig retinal pigment epithelium in vivo. Arch Ophthalmol 113:939–944
Del Priore LV, Kaplan HJ, Hornbeck R, Jones Z, Swinn M (1996) Retinal pigment epithelial debridement as a model for the pathogenesis and treatment of macular degeneration. Am J Ophthalmol 122:629–643
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR (2004) Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351:2805–2816
Grossniklaus HE, Green WR (2004) Choroidal neovascularization. Am J Ophthalmol 137:496–503
Grossniklaus HE, Hutchinson AK, Capone A Jr, Woolfson J, Lambert HM (1994) Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmology 101:1099–1111
Grossniklaus HE, Ling JX, Wallace TM et al (2002) Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 8:119–126
Grossniklaus HE, Martinez JA, Brown VB et al (1992) Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol 114:464–472
Grossniklaus HE, Miskala PH, Green WR et al (2005) Histopathologic and ultrastructural features of surgically excised subfoveal choroidal neovascular lesions: submacular surgery trials report no. 7. Arch Ophthalmol 123:914–921
He S, Ding Y, Zhou J et al (2005) Soluble EphB4 regulates choroidal endothelial cell function and inhibits laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci 46:4772–4779
Ivert L, Kong J, Gouras P (2003) Changes in the choroidal circulation of rabbit following RPE removal. Graefes Arch Clin Exp Ophthalmol 241:656–666
Kato A, Kimura H, Okabe K et al (2005) Suppression of laser-induced choroidal neovascularization by posterior sub-tenon administration of triamcinolone acetonide. Retina 25:503–509
Kiilgaard JF, Andersen MV, Wiencke AK et al (2005) A new animal model of choroidal neovascularization. Acta Ophthalmol Scand 83:697–704
Koh HJ, Freeman WR, Azen SP et al (2006) Effect of a novel octapeptide urokinase fragment, A6, on experimental choroidal neovascularization in the monkey. Retina 26:202–209
Kvanta A, Algvere PV, Berglin L, Seregard S (1996) Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37:1929–1934
Lassota N, Kiilgaard JF, Prause JU, la Cour M (2006) Correlation between clinical and histological features in a pig model of choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 244:394–398
Lauer AK, Wilson DJ, Klein ML (2000) Clinicopathologic correlation of fluorescein and indocyanine green angiography in exudative age-related macular degeneration. Retina 20:492–499
Lopez PF, Lambert HM, Grossniklaus HE, Sternberg P Jr (1993) Well-defined subfoveal choroidal neovascular membranes in age-related macular degeneration. Ophthalmology 100:415–422
Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR (1996) Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 37:855–868
Miller H, Miller B, Ishibashi T, Ryan SJ (1990) Pathogenesis of laser-induced choroidal subretinal neovascularization. Invest Ophthalmol Vis Sci 31:899–908
Miller H, Miller B, Ryan SJ (1986) Newly-formed subretinal vessels. Fine structure and fluorescein leakage. Invest Ophthalmol Vis Sci 27:204–213
Nishimura T, Zhu ZR, Ryan SJ (1990) Effects of sodium iodate on experimental subretinal neovascularization in the primate. Ophthalmologica 200:28–38
Ryan SJ (1982) Subretinal neovascularization. Natural history of an experimental model. Arch Ophthalmol 100:1804–1809
Ryan SJ (1980) Subretinal neovascularization after argon laser photocoagulation. Albrecht Von Graefes Arch Klin Exp Ophthalmol 215:29–42
Saxe SJ, Grossniklaus HE, Lopez PF, Lambert HM, Sternberg P Jr, L’Hernault N (1993) Ultrastructural features of surgically excised subretinal neovascular membranes in the ocular histoplasmosis syndrome. Arch Ophthalmol 111:88–95
Seregard S, Algvere PV, Berglin L (1994) Immunohistochemical characterization of surgically removed subfoveal fibrovascular membranes. Graefes Arch Clin Exp Ophthalmol 232:325–329
Silva RL, Saishin Y, Saishin Y et al (2005) Suppression and regression of choroidal neovascularization by polyamine analogues. Invest Ophthalmol Vis Sci 46:3323–3330
Spaide RF, Laud K, Fine HF et al (2006) Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 26:383–390
Warfvinge K, Kiilgaard JF, Lavik EB et al (2005) Retinal progenitor cell xenografts to the pig retina: morphologic integration and cytochemical differentiation. Arch Ophthalmol 123:1385–1393
Acknowledgements
This study was financially supported by The Danish Eye Research Foundation, Synoptik Fonden, Civilingeniør Lars Andersens Legat, Overlaerer Svend Hansen’s Fond, Fabrikant Einar Willumsens Mindelegat, Else og Mogens Wedell-Wedellsborgs Fond, Maskinfabrikant Jochum Jensen og hustru Mette Marie Jensens Mindelegat, Overlaege Poul M. Christiansens og hustrus fond, Mindefonden for laereinde Karen Svanekjær Ydes Fond, Civilingeniør Holger Rabitz og hustru Doris Mary, født Phillipps Mindelegat, Kleinsmed Svend Helge Arvid Schroeder og hustru Ketty Lydia Larsen Schroeders Fond.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no commercial interest in this study or in funding organizations. Full control of all primary data remains with the authors, and we agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review these data if requested.
Rights and permissions
About this article
Cite this article
Lassota, N., Kiilgaard, J.F., Prause, J.U. et al. Surgical induction of choroidal neovascularization in a porcine model. Graefes Arch Clin Exp Ophthalmol 245, 1189–1198 (2007). https://doi.org/10.1007/s00417-006-0518-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-006-0518-9