Abstract
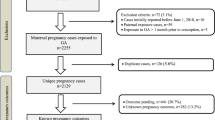
With the increasing incidence of multiple sclerosis (MS) in women and the earlier use of disease modifying therapy (DMT), issues surrounding DMT and pregnancy are a regular subject of discussion with regards to optimal management. Current recommendations are to withdraw DMT prior to conception, leaving patients exposed to an uncertain period of untreated disease. The objective of this study is to report preliminary experience on glatiramer acetate (GA) exposure through conception, pregnancy and the post-partum period in a series of 13 patients with previously highly active relapsing-remitting MS. This is a prospective observational case series. Fourteen pregnancies of 13 women resulted in 13 live births (one twin pregnancy), nine exposed to GA throughout pregnancy. There were no birth defects and treatment was well tolerated. No relapses occurred during pregnancy in those continuing on treatment. In conclusion, our early experience suggests that when considering the risks and benefits of treatment withdrawal prior to pregnancy, it may be reasonable to continue GA in those patients with previously highly active disease. Consideration should also be given to the initiation of a birth register, similar to such initiatives in epilepsy, to generate more robust safety data in this controversial area.
Similar content being viewed by others
References
Fernandes LRA, Fernandes RP, Fragoso YD, Lippi UG (2007) Multiple sclerosis and pregnancy. Einstein 5:173–176
Lee M, O’Brien P (2008) Pregnancy and multiple sclerosis. J Neurol Neurosurg Psychiatry 79:1308–1311
Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T, Pregnancy in Multiple Sclerosis Group (1998) Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med 339:285–291
Saraste M, Ryynanen J, Alanen A, Multanen J, Farkkila M, Kaaja R, Airas L (2006) Cerebrospinal fluid findings in MS patients before, during, and after pregnancy. J Neurol Neurosurg Psychiatry 77:1195–1196
Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, Confavreux C, The pregnancy in multiple sclerosis group (2004) Pregnancy and multiple sclerosis (The PRIMS study): clinical predictors of post-partum relapse. Brain 127:1353–1360
Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G (2005) The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology 65:807–811
Sandberg-Wollheim M, Frank D, Goodwin TM, Giesser B, Lopez-Bresnahan M, Stam-Moraga M et al (2005) Pregnancy outcomes during treatment with interferon beta-1a in patients with multiple sclerosis. Neurology 65:802–806
Putheti P, Soderstrom M, Link H, Huang YM (2003) Effects of glatiramer acetate (Copaxone) on CD4 + CD25 high T regulatory cells and their IL-10 production in multiple sclerosis. J Neuroimmunol 144:125–131
Coyle PK, Johnson K, Stark Y, Pardo L (2003) Pregnancy outcomes in patients with multiple sclerosis treated with glatiramer acetate (Copaxone). J Neurol Neurosurg Psychiatry 74:443 [poster]
Weber-Schoendorfer C, Schaefer C (2009) Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler 15:1037–1042
Ramtahal J, Jacob A, Das K, Boggild M (2006) Sequential treatment with glatiramer acetate after mitoxantrone is safe and can limit exposure to immunosuppression in very active, relapsing remitting MS. J Neurol 253:1160–1164
Miller A, DeAngelis T, Krieger S, Rooney-Crino A (2007) Use of glatiramer acetate during pregnancy: offering women a choice. Mult Scler 13: S7–S273 [abstract]
Ventura SJ, Martin JA, Curtin SC, Matthews TJ, Park MM (2000) Births: final data for 1998. Natl Vital Stat Rep 48:13–21
Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J et al (2004) The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 75:1575–1583
Acknowledgments
We would like to thank all the MS patients and their families for their participation in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salminen, H.J., Leggett, H. & Boggild, M. Glatiramer acetate exposure in pregnancy: preliminary safety and birth outcomes. J Neurol 257, 2020–2023 (2010). https://doi.org/10.1007/s00415-010-5652-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5652-y