Abstract.
Acute exacerbations may complicate the course of pregnancy and the postpartum period in patients with relapsing-remitting multiple sclerosis (RRMS). To evaluate relapse rate and the effect of immunomodulatory treatment with intravenous immunoglobulin (IVIg) during pregnancy and the postpartum period we retrospectively analysed the data of 108 pregnant RRMS patients. Group I patients were not treated, Group II patients were treated with IVIg 0.4 g/kg body weight/day for 5 consecutive days within the first week after delivery with additional booster doses of 0.4 g/kg body weight/day at 6 and 12 weeks postpartum (defined as 12 weeks after labor), and Group III patients were treated continuously with IVIg during gestation and the postpartum period (0.4 g/kg body weight/day for 5 consecutive days within the 6–8 weeks of gestation with additional booster doses of 0.4 g/kg body weight/day once every 6 weeks until 12 weeks postpartum). All patients underwent antenatal care and fetal ultrasonographic surveillance examinations. Relapse rate per woman per year during the pregnancy and the postpartum period as well as neonatal outcome data and IVIg related adverse events were analysed.
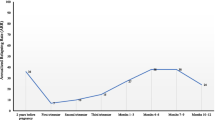
Relapse rate per woman per year for patients treated with IVIg for the whole pregnancy and postpartum period (Group III, N = 28) compared with the untreated Group I patients (N = 39) were as follows: first trimester 0.43 vs. 0.72, second trimester 0.15 vs. 0.61, third trimester 0.0 vs. 0.41, and postpartum period 0.28 vs.1.33 (p < 0.05). Patients treated with IVIg only during the postpartum period (Group II, N = 41) also showed a decrease in relapse rate compared with untreated Group I patients, 0.58 vs. 1.33 (p = 0.012). The mean maternal age, disease duration, gestational age at delivery and fetal delivery weight did not significantly differ between the three groups. Mode of delivery, obstetrical complications, the use of epidural analgesia and breast-feeding, did not affect postpartum relapse rate. No severe adverse events were associated with IVIg treatment either during the pregnancy or postpartum period for the patients and newborns.
We conclude that in RRMS patients IVIg treatment could be considered as an optional treatment to reduce the incidence of pregnancy and postpartum-related relapses. Further randomized double-blind studies are needed to confirm our findings.
Similar content being viewed by others
References
Achiron A, Mor F, Margalit R, Cohen IR, Lider O, Miron S (2000) Suppression of experimental autoimmune encephalomyelitis by intravenously administrated polyclonal immunoglobulins. J Autoimmun 15:323–330
Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R (1996) Intravenous immunoglobulin treatment in the prevention of childbirth associated acute exacerbations in multiple sclerosis—A pilot study. J Neurol 243:25–28
Birk K, Ford C, Smeltzer S, Ryan D, Miller R, Rudick RA (1990) The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol 47:738–742
Birk K, Smeltzer SC, Rudick R (1988) Pregnancy and multiple sclerosis. Semin Neurol 8:205–213
Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 339:285–291
Confavreux C, Vukusic S, Moreau T, Adeleine P (2000) Relapses and Progression of Disability in Multiple Sclerosis. N Engl J Med 343:1430–1438
Elenkov IJ, Chrousos GP (2002) Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966:290–303
Goodkin DE, Hertsgaard D, Rudick RA (1989) Exacerbation rates and adherence to disease type in a prospectively followed-up population with multiple sclerosis. Implications for clinical trials. Arch Neurol 46:1107–1112
Ito A, Bebo BF, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H (2001) Estrogen treatment downregulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol 167:542–552
Korn-Lubetzki I, Kahana E, Cooper G, Abramsky O (1984) Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol 16:229–231
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 5:121–127
Orvieto R, Achiron R, Rotstein Z, Noy S, Bar-Hava I, Achiron A (1999) Pregnancy and multiple sclerosis: a 2-year experience. Eur J Obstet Gynecol Reprod Biol 82:191–194
Pashov A, Bellon B, Kaveri SV, Kazatchkine MD (1997) A shift in encephalitogenic T cell cytokine pattern is associated with suppression of EAE by intravenous immunoglobulins (IVIg). Mult Scler 3:153–156
Poser CM, Paty DW, Scheinberg L, Mc-Donald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
Vassilev T, Kazatchkine MD (1997) Mechanisms of immunomodulatory action of intravenous immunoglobulin in autoimmune and systemic inflammatory diseases. Ther Apher 1:38–41
Weinshenker BG (1998) The natural history of multiple sclerosis: update 1998. Semin Neurol 18:301–307
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achiron, A., Kishner, I., Dolev, M. et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol 251, 1133–1137 (2004). https://doi.org/10.1007/s00415-004-0495-z
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00415-004-0495-z