Abstract
This study was designed to investigate the effect of Hochu-ekki-to (TJ-41), a Japanese herbal medicine, on the development of lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. ALI was induced in female BALB/c mice by the intranasal administration of 0.1 mg/kg LPS. The mice were divided into a group receiving normal feed and another group receiving feed mixed with TJ-41 at a dose of 1 g/kg/day for 8 weeks before LPS challenge. In the bronchoalveolar lavage fluid, the preadministration of TJ-41 caused significant reduction in the absolute number of total cells, neutrophils, and macrophages. The preadministration of TJ-41 significantly inhibited increases in the serum level of keratinocyte chemoattractant (KC), which is a murine chemotaxin for neutrophils that corresponds to human interleukin-8, with respect to its concentration at 24 h after LPS challenge. Furthermore, the histopathologic findings indicated that alveolitis with leukocyte infiltration in the alveolar space was less severe in the TJ-41-treated mice than in the control mice. These findings indicated that the preadministration of TJ-41 could show an inhibitory effect on ALI in this experimental murine system associated with the suppression of chemokine production.
Similar content being viewed by others
Introduction
Acute lung injury (ALI) and its severest form, acute respiratory distress syndrome (ARDS), are frequent complications in critically ill patients and are also responsible for significant morbidity and mortality [16]. An initiating event (e.g., sepsis, shock, trauma, multiple transfusions, pancreatitis) leads to the activation of an acute inflammatory response on a systemic level. One of the earliest manifestations is the activation of the pulmonary endothelium and macrophages (alveolar and interstitial), the upregulation of adhesion molecules, and the production of cytokines and chemokines that induce a massive sequestration of neutrophils within the pulmonary microvasculature. These cells transmigrate across the endothelium and epithelium into the alveolar space and then release a variety of cytotoxic and proinflammatory compounds, including proteolytic enzymes, reactive oxygen species (ROS) and nitrogen species, cationic proteins, lipid mediators, and additional inflammatory cytokines [6]. This phenomenon thereafter perpetuates a vicious cycle by recruiting additional inflammatory cells that in turn produce more cytotoxic mediators, ultimately leading to the occurrence of profound injury to the alveolocapillary membrane and respiratory failure. However, aside from the use of activated protein C in the subset of ALI/ARDS patients with sepsis [2], specific therapies are lacking, and the cascade of events leading to ALI and ARDS, once initiated, is much less amenable to specific treatment modalities. Therefore, new preventive measures against ALI/ARDS are eagerly awaited.
The herbal medicine known in Japan as Hochu-ekki-to (TJ-41) is known elsewhere by its Chinese name, bu-zhong-yi-qi-tang. TJ-41 is composed of ten species of medicinal plants and is used for chronic diseases or weakness after illness. Recent studies have shown that TJ-41 has been widely used to treat patients with certain immune-related diseases. TJ-41 suppresses the development of collagen-induced arthritis, redistributes the population of lymphocytes in lymph nodes and blood, and inhibits interleukin (IL)-6 and tumor necrosis factor α (TNFα) secretion in mice with collagen-induced arthritis [4]. The administration of TJ-41 decreased bronchoalveolar lavage fluid (BALF) concentrations of IL-1α, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) but not of TNFα or interferonγ (IFNγ), and it was found to increase the survival rate after influenza virus infection in mice [9]. Moreover, TJ-41 contains Glycyrrhizae radix, a flavonoid that has been shown to inhibit human neutrophil NADPH oxidase and impair neutrophil response [12, 14]. Wang et al. [15] reported that ROS produced by NADPH oxidase activation in neutrophils plays a major role in mediating sepsis-induced ALI, and the NADPH oxidase inhibitor attenuates sepsis-induced lung injury in guinea pigs. These findings suggest that TJ-41 may suppress ROS and proinflammatory cytokine production. In this article we investigate the suppressive effect of TJ-41 on the development of lipopolysaccharide (LPS)-induced ALI in mice.
Materials and Methods
Preparation of TJ-41
TJ-41 was authenticated and provided by Tsumura Co., Ltd. (Tokyo, Japan). The crude drug composition of TJ-41 is given in Table 1. The mixture of crude drugs was extracted with 600 ml of water at 100°C for 1 h. The decoction was filtered and then lyophilized to obtain a powdered extract. The yield of the extract is also noted in Table 1. The doses of 0.15, 0.74, and 1.47 g/kg body weight were roughly equivalent to 1, 5, and 10 times the daily human dose, respectively.
Animals and Drug Administration
Female BALB/c mice, 3 weeks of age, were obtained from Japan SLC (Tochigi, Japan) and housed in the animal facility of the Jichi Medical School. All animal experiments were conducted in accordance with the principles stated in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23, National Institutes of Health, Bethesda, MD, 1985). The dose of TJ-41 was adjusted to 1 g/kg body weight/day by mixing it with normal feed. The mice then either consumed normal feed (Control) or feed mixed with TJ-41 from 8 weeks before LPS challenge until the end of the study.
LPS-Induced ALI Model
After the mice were anesthetized by the intraperitoneal administration of 0.2 ml of 10% pentobarbital sodium solution (Abbott Laboratories, North Chicago, IL), 0.1 mg/kg LPS in 0.06 ml of saline was administered intranasally as previously described [13]. BALF was sampled at each time point, according to a standard protocol [7]. Briefly, the trachea was cannulated with a polyethylene tube, and then the lungs were lavaged four times with 0.7 ml of sterile phosphate-buffered saline. Total cell counts were determined from BALF using a hemocytometer. Blood samples were also obtained from the right atrium at each time point. After centrifugation at 3000g for 10 min at 4°C, the serum was frozen and stored at −80°C until it was assayed.
Assays for Cytokines and Lipid Hydroperoxide
The serum levels of keratinocyte chemoattractant (KC) and GM-CSF were determined by a sandwich enzyme immunoassay using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, and Biosource International Inc., Camarillo, CA, respectively) according to the manufacturers’ instructions. The concentrations of lipid hydroperoxide (LPO) in BALF and serum were measured as an indicator of oxidative stress using the Lipid Hydroperoxide Assay kit (Cayman Chemical, Ann Arbor, MI).
Morphologic Evaluation
Histopathologic evaluation was performed on animals that were not subjected to BAL. Thereafter, both lungs were removed and inflated with 10% formaldehyde neutral buffer solution, and longitudinal tissue sections were stained with hematoxylin & eosin.
Statistical Analysis
Data were expressed as the mean ± SEM. Multiple comparisons were carried out by the Fisher protected least-significant-differences method followed by the post hoc test. Differences between two variables were assessed with the Mann-Whitney U test. p < 0.05 was considered significant.
Results
Effects of TJ-41 on Changes in Body Weight in BALB/c Mice
The results demonstrated no effect on body weight by TJ-41. As shown in Figure 1, no significant weight gain or loss was observed in mice that consumed feed mixed with TJ-41 for 8 weeks (TJ-41: 11wk) compared with those that consumed normal feed for 8 weeks (Control: 11wk) (p = 0.890; Control: 21.1 ± 0.2 g vs. TJ-41: 21.2 ± 0.2 g).
Effects of TJ-41 on changes in body weight. There was no significant weight gain or loss in the mice that received feed mixed with TJ-41 for 8 weeks (TJ-41: 11wk) compared with mice that received ordinary feed for 8 weeks (Control: 11wk). Data are presented as the mean ± SEM (n = 25–27 in each group)
Effects of TJ-41 on BALF Cell Analysis
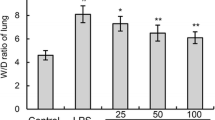
The effect of TJ-41 on lung inflammation in BALB/c mice was examined using an LPS-induced ALI model, which has been extensively characterized. A total of 1g/kg/day of TJ-41 was administered for 8 weeks, and then LPS was instilled intranasally. The number of total cells in BALF in TJ-41-treated mice at 72 h after LPS challenge significantly decreased compared with those in untreated mice [p = 0.006; Control: (12.2 ± 1.1) × 105/ml vs. TJ-41: (8.4 ± 1.0) × 105/ml] (Fig. 2A). Similarly, the preadministration of TJ-41 caused a significant reduction in the neutrophils in BALF at 72 h after LPS challenge [p < 0.001; Control: (5.7 ± 0.8) × 105/ml vs. TJ-41: (2.0 ± 0.4) × 105/ml] (Fig. 2B). At 120 h after LPS challenge, the preadministration of TJ-41 caused a significant reduction in the macrophages in BALF [p = 0.043; Control: (5.6 ± 1.1) × 105/ml vs. TJ-41: (3.4 ± 0.3) × 105/ml] (Fig. 2C); however, no difference was seen in the lymphocytes in BALF in TJ-41-treated mice compared with those in untreated mice (Fig. 2D).
Effects of TJ-41 on BALF cell analysis in a LPS-induced acute lung injury model. TJ-41 was administered for 8 weeks before LPS challenge and then was continued to 120 h after LPS challenge. BALF was collected and cell differentiation was determined before and at the indicated times after the intranasal administration of LPS, as described in Materials and Methods. Data are presented as the mean ± SEM (n = 5–10 in each group). *p < 0.05 compared with the control mice at each time point after LPS challenge
Effects of TJ-41 on the Amount of Lipid Hydroperoxide in BALF and Serum
Although reports have demonstrated that TJ-41 suppresses ROS [12, 14], the results of our study did not demonstrate an inhibitory effect on ROS by TJ-41. The serum levels of LPO in TJ-41-treated mice at 72 h after LPS challenge did not differ from those in untreated mice (p = 0.903; Control: 3.34 ± 0.29 nmol/ml vs. TJ-41: 3.28 ± 0.18 nmol/ml). Similar to serum, no difference was observed in the BALF levels of LPO in TJ-41-treated mice comparee with those in untreated mice at 72 h after LPS challenge (p = 0.706; Control: 1.16 ± 0.26 nmol/ml vs. TJ-41: 1.08 ± 0.25 nmol/ml).
Effects of TJ-41 on KC and GM-CSF Concentration in Serum
The serum levels of KC, which is a murine chemotaxin for neutrophils that corresponds to human IL-8, peaked at 24 h after LPS challenge and thereafter rapidly decreased (Fig. 3). This elevation was significantly attenuated by the preadministration of TJ-41 (p = 0.002; Control: 390.17 ± 79.37 pg/ml vs. TJ-41: 264.96 ± 47.02 pg/ml) (Fig. 3). On the other hand, the serum levels of GM-CSF in TJ-41-treated mice at 72 h after LPS challenge tended to be lower than those in untreated mice (p = 0.172; Control: 33.53 ± 7.64 pg/ml vs. TJ-41: 24.28 ± 9.06 pg/ml).
Effects of TJ-41 on KC concentration in the serum in a LPS-induced acute lung injury model. TJ-41 was administered for 8 weeks before LPS challenge and then was continued to 120 h after LPS challenge. Serum was collected before and at the indicated times after the intranasal administration of LPS, as described in Materials and Methods. Data are presented as the mean ± SEM (n = 5–10 in each group). *p < 0.05 compared with control mice at each time point after LPS challenge
Effects of TJ-41 on Histopathologic Changes
After LPS challenge, edematous thickening of alveolar septa with the infiltration of neutrophils and macrophages and a few lymphocytes were observed (Fig. 4B-D). Neutrophils also infiltrated the alveolar space (Fig. 4B-D). At 24 h after LPS challenge, the alveolar thickening and cellular infiltration became most severe (Fig. 4B). After LPS challenge, alveolitis and neutrophil infiltration in the alveolar space was less severe in TJ-41-treated mice than in control mice (Fig. 4F-H).
Effects of TJ-41 on histopathologic changes in LPS-induced acute lung injury model. Representative pictures of lung tissue specimens from mice that received ordinary feed for 8 weeks (A–D) and those that received feed mixed with TJ-41 for 8 weeks (E–H). Histopathology was compared between the two groups at 0 h (A, E), 24 h (B, F), 72 h (C, G), and 96 h (D, H) after LPS challenge. No effect on the lung tissue findings was observed in mice that consumed feed mixed with TJ-41 for 8 weeks (E) compared with mice that consumed ordinary feed for 8 weeks (A). Alveolar thickening with the cellular infiltration of monocytes, neutrophils, and a few lymphocytes and neutrophil infiltration in the alveolar space are seen, particularly at 24 h after LPS challenge (B, F); however, these features were less severe in TJ-41-treated mice (F) [original × 100]
Discussion
Although Ohtake et al. [10, 11] demonstrated that the oral administration of Sho-saiko-to ameliorated lung injury induced by intranasal LPS instillation in BALB/c mice, Ishizaki et al. [5] showed that Sho-saiko-to tended to induce acute pneumonitis by allergic-immunologic mechanisms in patients with chronic active hepatitis. Indeed, in some cases, Shosaiko-to leads to interstitial pneumonia, especially when used together with IFN, and some cases become serious. Therefore, we have tried to find other Japanese herbal medicines that have a protective effect on ALI/ARDS without such adverse effects. In this study we examined the preventive effects of Hochu-ekki-to on an LPS-induced ALI model. In BALF, the preadministration of TJ-41 caused a significant reduction in the total cells, neutrophils, and macrophages. The preadministration of TJ-41 significantly inhibited the increase in serum KC concentration at 24 h after LPS challenge. Furthermore, the histopathologic findings indicated that alveolitis was less severe in TJ-41-treated mice than in control mice. These findings suggest that the preadministration of TJ-41 could be expected to show an inhibitory effect on ALI/ARDS via the modulation of proinflammatory cytokines.
Clinical and experimental studies have provided circumstantial evidence of the occurrence of neutrophil-mediated injury in ALI/ARDS. Histologic studies of human lung specimens obtained early in the course of the disorder show marked accumulation of neutrophils [16]. Neutrophils predominated in pulmonary edema fluid and BALF obtained from the affected patients, and many animal models of ALI are neutrophil-dependent [16]. In the acute phase of ALI/ARDS, neutrophils are shown to adhere to the injured capillary endothelium and then migrate through the interstitium into the air space, which is filled with protein-rich edema fluid. In the air space, alveolar macrophages secret cytokines, IL-1, IL-6, IL-8, and IL-10 and TNFα, all of which act locally to stimulate chemotaxis and activate neutrophils [16]. As shown in Figure 3, the serum levels of KC, which is a chemotaxin for neutrophils similar to human IL-8, in LPS-induced ALI mice receiving TJ-41 were significantly lower than those in control mice at 24 h after LPS challenge. These reductions may have contributed to the decreased neutrophil count in BALF of the LPS-induced ALI model treated with TJ-41 (Fig. 2B). It is of interest that KC production was affected by TJ-41, and a study of its suppression mechanism is now underway.
As shown in Figure 2C, the preadministration of TJ-41 caused a significant reduction in the macrophages in BALF of the LPS-induced ALI model. In one study of BALF from patients with ALI/ARDS, GM-CSF has been shown to increase [8]. GM-CSF is thus considered to play a role in the proliferation, differentiation, and survival of macrophages, while also regulating the expansion and maturation of their precursors [1]. The serum levels of GM-CSF in TJ-41-treated mice at 72 h after LPS challenge tend to be lower than those in untreated mice but the difference is not significant. The administration of TJ-41 decreased BALF concentrations of GM-CSF after influenza virus infection in mice [9]. We could not detect GM-CSF in the BALF by ELISA at any time after LPS challenge in our LPS-induced ALI model (data not shown). Further analysis is thus required to investigate the mechanism of the suppressive effect of TJ-41 on macrophage infiltration in the lungs of LPS-induced injury.
One possible explanation for the preventive effect of TJ-41 on ALI in this mouse model is its antioxidant effect. We expect that TJ-41 suppresses ROS production in an LPS-induced ALI model. We measured the amount of LPO in the BALF and serum, which was an indicator of oxidative stress [3]. The results of this study, however, demonstrated that the serum and BALF levels of LPO did not decrease in TJ-41-treated mice. These findings suggest that TJ-41 might have only a slight effect on ROS production in the lungs of the LPS-induced ALI model.
In summary, the results of this study proved that the preadministration of TJ-41 reduced LPS-induced ALI in BALB/c mice. The ameliorating effects of neutrophil infiltration on the lung seemed to be due to reduced KC production by TJ-41. These findings suggest that the preadministration of TJ-41 may therefore modulate the process of ALI/ARDS via regulating proinflammatory cytokine production. However, the precise mechanism by which TJ-41 modulates proinflammatory cytokine production remains obscure. Further studies are required to elucidate this issue.
References
Barreda DR, Hanington PC, Belosevic M (2004) Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol 28:509–554
Bernard GR, Vincent JL, Laterre PF, et al. (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Hagiwara SI, Ishii Y, Kitamura S (2000) Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162:225–231
Hai le X, Kogure T, Niizawa A, et al. (2002) Suppressive effect of hochu-ekki-to on collagen induced arthritis in DBA1J mice. J Rheumatol 29:1601–1608
Ishizaki T, Sasaki F, Ameshima S, et al. (1996) Pneumonitis during interferon and/or herbal drug therapy in patients with chronic active hepatitis. Eur Respir J 12:2691–2696
Lee WL, Downey GP (2001) Neutrophil activation and acute lung injury. Curr Opin Crit Care 7:1–7
Löhning M, Stroehmann A, Coyle AJ, et al. (1998) T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A 95:6930–6935
Matute-Bello G, Liles WC, Radella F 2nd, et al. (1997) Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 156:1969–1977
Mori K, Kido T, Daikuhara H, et al. (1999) Effect of Hochu-ekki-to (TJ-41), a Japanese herbal medicine, on the survival of mice infected with influenza virus. Antiviral Res 44:103–111
Ohtake N, Suzuki R, Daikuhara H, et al. (2000) Modulation of lung local immune responses by oral administration of a herbal medicine Sho-saiko-to. Int J Immunopharmacol 22:419–430
Ohtake N, Nakai Y, Yamamoto M, et al. (2002) The herbal medicine Shosaiko-to exerts different modulating effects on lung local immune responses among mouse strains. Int Immunopharmacol 2:357–366
Pagonis C, Tauber AI, Pavlotsky N, Simons ER (1986) Flavonoid impairment of neutrophil response. Biochem Pharmacol 35:237–245
Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH (1997) A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods 202:49–57
Tauber AI, Fay JR, Marletta MA (1984) Flavonoid inhibition of the human neutrophil NADPH-oxidase. Biochem Pharmacol 33:1367–1369
Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA (1994) Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med 150:1449–1452
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Acknowledgments
The authors thank Mrs. Tomoko Ikahata for her valuable assistance. This study was supported by a grant awarded from the Ministry of Health, Labour and Welfare of Japan to the Long Life Science Research Group (H17-Long Life-027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tajima, S., Bando, M., Yamasawa, H. et al. Preventive Effect of Hochu-ekki-to on Lipopolysaccharide-Induced Acute Lung Injury in BALB/c Mice. Lung 184, 318–323 (2006). https://doi.org/10.1007/s00408-006-0018-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-006-0018-z