Abstract
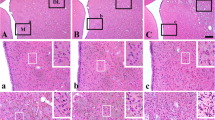
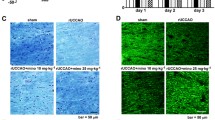
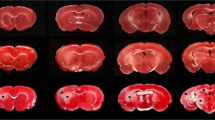
Though cerebral white matter injury is a frequently described phenomenon in aging and dementia, the cause of white matter lesions has not been conclusively determined. Since the lesions are often associated with cerebrovascular risk factors, ischemia emerges as a potential condition for the development of white matter injury. In the present study, we induced experimental cerebral hypoperfusion by permanent, bilateral occlusion of the common carotid arteries of rats (n=6). A sham-operated group served as control (n=6). Thirteen weeks after the onset of occlusion, markers for astrocytes, microglia, and myelin were found to be labeled by means of immunocytochemistry in the corpus callosum, the internal capsule, and the optic tract. The ultrastructural integrity and oligodendrocyte density in the optic tract were investigated by electron microscopy. Quantitative analysis revealed that chronic cerebral hypoperfusion caused mild astrogliosis in the corpus callosum and the internal capsule, while astrocytic disintegration in the optic tract increased by 50%. Further, a ten-fold increase in microglial activation and a nearly doubled oligodendrocyte density were measured in the optic tract of the hypoperfused rats as compared with the controls. Finally, vacuolization and irregular myelin sheaths were observed at the ultrastructural level in the optic tract. In summary, the rat optic tract appears to be particularly vulnerable to ischemia, probably because of the rat brain’s angioarchitecture. Since the detected glial changes correspond with those reported in vascular and Alzheimer dementia, this model of cerebral hypoperfusion may serve to characterize the causal relationship between ischemia and white matter damage.




Similar content being viewed by others
References
Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O’Brien JT (2000) MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry 15(10):911–916
Barkhof F, Scheltens P (2002) Imaging of white matter lesions. Cerebrovasc Dis 13 [Suppl 2]:21–30
Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J (2003) White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol 60(3):393–398
Chan SO, Guillery RW (1994) Changes in fiber order in the optic nerve and tract of rat embryos. J Comp Neurol 344(1):20–32
Chelvanayagam DK, Dunlop SA, Beazley LD (1998) Axon order in the visual pathway of the quokka wallaby J Comp Neurol 390(3):333–341
Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA (2000) Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res 859(1):96–103
de Groot CJ, Hulshof S, Hoozemans JJ, Veerhuis R (2001) Establishment of microglial cell cultures derived from postmortem human adult brain tissue: immunophenotypical and functional characterization. Microsc Res Tech 54(1):34–39
Englund E (1998) Neuropathology of white matter changes in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord 9 [Suppl 1]:6–12
Farkas E, de Wilde MC, Kiliaan AJ, Meijer J, Keijser JN, Luiten PG (2002) Dietary long chain PUFAs differentially affect hippocampal muscarinic 1 and serotonergic 1A receptors in experimental cerebral hypoperfusion. Brain Res 954(1):32–41
Farkas IG, Czigner A, Farkas E, Dobo E, Soos K, Penke B, Endresz V, Mihaly A (2003) Beta-amyloid peptide-induced blood-brain barrier disruption facilitates T-cell entry into the rat brain. Acta Histochem 105(2):115–125
Fazekas F, Schmidt R, Kleinert R, Kapeller P, Roob G, Flooh E (1998) The spectrum of age-associated brain abnormalities: their measurement and histopathological correlates. J Neural Transm [Suppl 53]:31–39
Horvath KM, Abraham IM, Harkany T, Meerlo P, Bohus BG, Nyakas C, Luiten PGM (2000) Postnatal treatment with ACTH-(4–9) analog ORG 2766 attenuates N-methyl-D-aspartate-induced excitotoxicity in rat nucleus basalis in adulthood. Eur J Pharmacol 405(1–3):33–42
Ihara M, Tomimoto H, Kinoshita M, Oh J, Noda M, Wakita H, Akiguchi I, Shibasaki H (2001) Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab 21(7):828–834
Irving EA, Bentley DL, Parsons AA (2001) Assessment of white matter injury following prolonged focal cerebral ischaemia in the rat. Acta Neuropathol (Berl) 102(6):627–635
Kobari M, Meyer JS, Ichijo M, Oravez WT (1990) Leukoaraiosis: correlation of MR and CT findings with blood flow, atrophy, and cognition. Am J Neuroradiol 11(2):273–281
Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y (2002) Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathol Appl Neurobiol 28(3):238–251
Lees GJ (1993) The possible contribution of microglia and macrophages to delayed neuronal death after ischemia. J Neurol Sci 114(2):119–122
Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J (2001) Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia 35(1):72–79
Martin JA, Craft DK, Su JH, Kim RC, Cotman CW (2001) Astrocytes degenerate in frontotemporal dementia: possible relation to hypoperfusion. Neurobiol Aging 22(2):195–207
Masumura M, Hata R, Nagai Y, Sawada T (2001) Oligodendroglial cell death with DNA fragmentation in the white matter under chronic cerebral hypoperfusion: comparison between normotensive and spontaneously hypertensive rats. Neurosci Res 39(4):401–412
Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H (1998) Chronic cerebral hypoperfusion-induced neuropathological changes in rats. Nihon Shinkei Seishin Yakurigaku Zasshi 18(5):181–188
Paxinos G, Watson C. (1986) The rat brain in stereotactic coordinates, 2nd edn, Academic Press, New York
Peters A (2002) The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 31(8–9):581–593
Rosenberg GA, Sullivan N, Esiri MM (2001) White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke 32(5):1162–1168
Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W (1995) Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology 45(5):883–888
Scremin OU (1995) Cerebral Vascular System. In: Paxinos G, ed, The rat nervous system, 2nd edn, Academic Press, New York, pp 3–35
Stoll G, Jander S, Schroeter M (1998) Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 56(2):149–171
Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH (2002) Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma 19(1):85–98
Takizawa S, Fukuyama N, Hirabayashi H, Kohara S, Kazahari S, Shinohara Y, Nakazawa H (2003) Quercetin, a natural flavonoid, attenuates vacuolar formation in the optic tract in rat chronic cerebral hypoperfusion model. Brain Res 980(1):156–160
Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ (1997) Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging 18(6):609–615
Thomas AJ, Perry R, Barber R, Kalaria RN, O’Brien JT (2002) Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann N Y Acad Sci 977:333–339
Tomimoto H, Akiguchi I, Wakita H, Suenaga T, Nakamura S, Kimura J (1997) Regressive changes of astroglia in white matter lesions in cerebrovascular disease and Alzheimer’s disease patients. Acta Neuropathol (Berl) 94(2):146–152
Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, Kinoshita M, Shibasaki H (2003) Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol (Berl) 106(6):527–534
Wakita H, Tomimoto H, Akiguchi I, Kimura J (1994) Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol (Berl) 87(5):484–492
Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, McGeer PL (2002) Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res 924(1):63–70
Zeman W, Innes JRM (1963) Craigie’s Neuroanatomy of the Rat, Academic Press, New York
Zhang ZG, Bower L, Zhang RL, Chen S, Windham JP, Chopp M (1999) Three-dimensional measurement of cerebral microvascular plasma perfusion, glial fibrillary acidic protein and microtubule associated protein-2 immunoreactivity after embolic stroke in rats: a double fluorescent labeled laser-scanning confocal microscopic study. Brain Res 844(1–2):55–66
Acknowledgements
This project was supported by grants F042803 and T32566 from the Hungarian Scientific Research Fund (OTKA), a Bolyai János Research Scholarship of the Hungarian Academy of Sciences to EF, and a Visiting Scholar grant from the Royal Netherlands Academy of Sciences to EF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farkas, E., Donka, G., de Vos, R.A.I. et al. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol 108, 57–64 (2004). https://doi.org/10.1007/s00401-004-0864-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0864-9