Abstract
Purpose
Many health benefits have been attributed to tea (Camellia sinensis (L.)), and tea infusions are used as dietary agent and included in food supplements. Herein, we report the effect of a white tea (WTEA) extract in Sertoli cell (SC) metabolism. The SC is responsible for the nutritional support of the developing germ cells.
Methods
An aqueous WTEA extract was prepared and analyzed by 1H-NMR. Rat SCs were cultured with or without the WTEA extract. mRNA and protein levels of glucose transporters (GLUT1 and GLUT3), phosphofructokinase, lactate dehydrogenase (LDH) and monocarboxylate transporter 4 were determined by qPCR and western blot. LDH activity was assessed and metabolite production/consumption determined by 1H-NMR.
Results
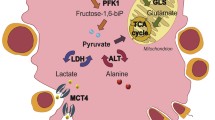
WTEA-exposed SCs presented decreased protein and mRNA levels of GLUT1 and decreased glucose uptake. However, intracellular LDH activity was increased and SC lactate production was stimulated by the presence of the WTEA extract. Interestingly, alanine production was also found to be stimulated in WTEA extract-exposed SCs.
Conclusion
WTEA extract altered the glycolytic profile of cultured SCs, stimulating lactate production. Since lactate is used as metabolic substrate and has an anti-apoptotic effect in the developing germ cells, the supplementation with WTEA extract may be advantageous to improve male reproductive health.



Similar content being viewed by others
References
Cabrera C, Artacho R, Gimenez R (2006) Beneficial effects of green tea: a review. J Am Coll Nutr 25(2):79–99. doi:10.1080/07315724.2006.10719518
Dufresne CJ, Farnworth ER (2001) A review of latest research findings on the health promotion properties of tea. J Nutr Biochem 12(7):404–421. doi:10.1016/S0955-2863(01)00155-3
Higdon JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43(1):89–143. doi:10.1080/10408690390826464
Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM (2009) Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J Am Coll Nutr 28(4):355–361. doi:10.1080/07315724.2009.10718097
Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, Miyamoto Y, Shimizu M (2000) Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem 48(11):5618–5623. doi:10.1021/jf0006832
Hodgson AB, Randell RK, Boon N, Garczarek U, Mela DJ, Jeukendrup AE, Jacobs DM (2013) Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem 24(1):325–334. doi:10.1016/j.jnutbio.2012.06.017
Van Dorsten FA, Daykin CA, Mulder TP, Van Duynhoven JP (2006) Metabonomics approach to determine metabolic differences between green tea and black tea consumption. J Agric Food Chem 54(18):6929–6938. doi:10.1021/jf061016x
Sugiyama A, Chiba M, Nakagami T, Kawano S, Sanada Y, Tajiri T, Toki A (2012) Beneficial effects of (–)-epigallocatechin gallate on ischemia-reperfusion testicular injury in rats. J Pediatr Surg 47(7):1427–1432. doi:10.1016/j.jpedsurg.2012.01.069
Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ (2002) Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol Sci 69(2):354–361. doi:10.1093/toxsci/69.2.354
Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF (2012) Metabolic regulation is important for spermatogenesis. Nat Rev Urol 9(6):330–338. doi:10.1038/nrurol.2012.77
Alves MG, Rato L, Carvalho RA, Moreira PI, Socorro S, Oliveira PF (2013) Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell Mol Life Sci 70(5):777–793. doi:10.1007/s00018-012-1079-1
Rato L, Alves MG, Socorro S, Carvalho RA, Cavaco JE, Oliveira PF (2012) Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci Rep 32(1):61–69. doi:10.1042/bsr20110030
Alves MG, Socorro S, Silva J, Barros A, Sousa M, Cavaco JE, Oliveira PF (2012) In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17beta-estradiol and suppressed by insulin deprivation. Biochim Biophys Acta 1823(8):1389–1394. doi:10.1016/j.bbamcr.2012.06.002
Oliveira PF, Alves MG, Rato L, Laurentino S, Silva J, Sa R, Barros A, Sousa M, Carvalho RA, Cavaco JE, Socorro S (2012) Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim Biophys Acta 1820(2):84–89. doi:10.1016/j.bbagen.2011.11.006
Oliveira PF, Alves MG, Rato L, Silva J, Sa R, Barros A, Sousa M, Carvalho RA, Cavaco JE, Socorro S (2011) Influence of 5alpha-dihydrotestosterone and 17beta-estradiol on human Sertoli cells metabolism. Int J Androl 34(6 Pt 2):e612–e620. doi:10.1111/j.1365-2605.2011.01205.x
Alves MG, Neuhaus-Oliveira A, Moreira PI, Socorro S, Oliveira PF (2013) Exposure to 2,4-dichlorophenoxyacetic acid alters glucose metabolism in immature rat Sertoli cells. Reprod Toxicol 38C:81–88. doi:10.1016/j.reprotox.2013.03.005
Sharangi A (2009) Medicinal and therapeutic potentialities of tea (Camellia sinensis L.): a review. Food Res Int 42(5):529–535. doi:10.1016/j.foodres.2009.01.007
Rusak G, Komes D, Likić S, Horžić D, Kovač M (2008) Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem 110(4):852–858. doi:10.1016/j.foodchem.2008.02.072
Loew D, Kaszkin M (2002) Approaching the problem of bioequivalence of herbal medicinal products. Phytother Res 16(8):705–711. doi:10.1002/ptr.1248
Zivkovic D, Hadziselimovic F (2009) Development of Sertoli cells during mini-puberty in normal and cryptorchid testes. Urol Int 82(1):89–91. doi:10.1159/000176032
Simoes VL, Alves MG, Martins AD, Dias TR, Rato L, Socorro S, Oliveira PF (2013) Regulation of apoptotic signaling pathways by 5alpha-dihydrotestosterone and 17beta-estradiol in immature rat Sertoli cells. J Steroid Biochem Mol Biol 135C:15–23. doi:10.1016/j.jsbmb.2012.11.019
Alves MG, Machado NG, Sardao VA, Carvalho RA, Oliveira PJ (2011) Anti-apoptotic protection afforded by cardioplegic celsior and histidine buffer solutions to hearts subjected to ischemia and ischemia/reperfusion. J Cell Biochem 112(12):3872–3881. doi:10.1002/jcb.23320
Alves MG, Oliveira PJ, Carvalho RA (2011) Substrate selection in hearts subjected to ischemia/reperfusion: role of cardioplegic solutions and gender. NMR in Biomed 24(9):1029–1037. doi:10.1002/nbm.1640
Galardo MN, Riera MF, Pellizzari EH, Chemes HE, Venara MC, Cigorraga SB, Meroni SB (2008) Regulation of expression of Sertoli cell glucose transporters 1 and 3 by FSH, IL1 beta, and bFGF at two different time-points in pubertal development. Cell Tissue Res 334(2):295–304. doi:10.1007/s00441-008-0656-y
Dias TR, Tomás G, Teixeira N, Alves MG, Oliveira PF, Silva BM (2013) White tea (Camellia Sinensis (L.)): antioxidant properties and beneficial health effects. Int J Food Sci Nutr Diet 2:1–15
Kaszkin M, Beck KF, Eberhardt W, Pfeilschifter J (2004) Unravelling green tea’s mechanisms of action: more than meets the eye. Mol Pharmacol 65(1):15–17. doi:10.1124/mol.65.1.15
Williams SN, Shih H, Guenette DK, Brackney W, Denison MS, Pickwell GV, Quattrochi LC (2000) Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem Biol Interact 128(3):211–229. doi:10.1016/S0009-2797(00)00204-0
Monsees TK, Franz M, Gebhardt S, Winterstein U, Schill WB, Hayatpour J (2000) Sertoli cells as a target for reproductive hazards. Andrologia 32(4–5):239–246. doi:10.1046/j.1439-0272.2000.00391.x
Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, Delgado-Lopez F, Guaiquil VH, Vera JC, Concha II (1998) Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem 71(2):189–203
Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB (2009) Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am J Physiol Endocrinol Metab 297(4):E907–E914. doi:10.1152/ajpendo.00235.2009
Shimizu M, Kobayashi Y, Suzuki M, Satsu H, Miyamoto Y (2000) Regulation of intestinal glucose transport by tea catechins. BioFactors 13(1–4):61–65. doi:10.1002/biof.5520130111
Juhel C, Armand M, Pafumi Y, Rosier C, Vandermander J, Lairon D (2000) Green tea extract (AR25) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J Nutr Biochem 11(1):45–51. doi:10.1016/S0955-2863(99)00070-4
Naz S, Siddiqi R, Dew TP, Williamson G (2011) Epigallocatechin-3-gallate inhibits lactase but is alleviated by salivary proline-rich proteins. J Agric Food Chem 59(6):2734–2738. doi:10.1021/jf103072z
Kim JW, Dang CV (2005) Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30(3):142–150. doi:10.1016/j.tibs.2005.01.005
Sundaram R, Naresh R, Shanthi P, Sachdanandam P (2013) Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine 20(7):577–584. doi:10.1016/j.phymed.2013.01.006
Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF (2013) Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta 1832(5):626–635. doi:10.1016/j.bbadis.2013.01.011
Rato L, Alves MG, Socorro S, Carvalho RA, Cavaco JE, Oliveira PF (2012) Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci Rep 32(1):61–69. doi:10.1042/bsr20110030
Martins AD, Alves MG, Simoes VL, Dias TR, Rato L, Moreira PI, Socorro S, Cavaco JE, Oliveira PF (2013) Control of Sertoli cell metabolism by sex steroid hormones is mediated through modulation in glycolysis-related transporters and enzymes. Cell Tissue Res 354(3):861–868. doi:10.1007/s00441-013-1722-7
Jutte NH, Eikvar L, Levy FO, Hansson V (1985) Metabolism of palmitate in cultured rat Sertoli cells. J Reprod Fertil 73(2):497–503. doi:10.1530/jrf.0.0730497
Kaiser GR, Monteiro SC, Gelain DP, Souza LF, Perry ML, Bernard EA (2005) Metabolism of amino acids by cultured rat Sertoli cells. Metabolism 54(4):515–521. doi:10.1016/j.metabol.2004.11.005
Xiong W, Wang H, Wu H, Chen Y, Han D (2009) Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 137(3):469–479. doi:10.1530/rep-08-0343
Slaughter GR, Means AR (1983) Follicle-stimulating hormone activation of glycogen phosphorylase in the Sertoli cell-enriched rat testis. Endocrinology 113(4):1476–1485. doi:10.1210/endo-113-4-1476
Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK (2002) Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277(38):34933–34940. doi:10.1074/jbc.M204672200
O’Donnell JM, Kudej RK, LaNoue KF, Vatner SF, Lewandowski ED (2004) Limited transfer of cytosolic NADH into mitochondria at high cardiac workload. Am J Phys 286(6):H2237–H2242. doi:10.1152/ajpheart.01113.2003
Ren F, Zhang S, Mitchell SH, Butler R, Young CY (2000) Tea polyphenols down-regulate the expression of the androgen receptor in LNCaP prostate cancer cells. Oncogene 19(15):1924–1932. doi:10.1038/sj.onc.1203511
Sukhthankar M, Alberti S, Baek SJ (2010) (–)-Epigallocatechin-3-gallate (EGCG) post-transcriptionally and post-translationally suppresses the cell proliferative protein TROP2 in human colorectal cancer cells. Anticancer Res 30(7):2497–2503
Courtens JL, Ploen L (1999) Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol Reprod 61(1):154–161
Erkkila K, Aito H, Aalto K, Pentikainen V, Dunkel L (2002) Lactate inhibits germ cell apoptosis in the human testis. Molec Human Reprod 8(2):109–117. doi:10.1093/molehr/8.2.109
Acknowledgments
This work was supported by the “Fundação para a Ciência e a Tecnologia”—FCT (PTDC/QUI-BIQ/121446/2010 and PEst-C/SAU/UI0709/2011) co-funded by Fundo Europeu de Desenvolvimento Regional—FEDER via Programa Operacional Factores de Competitividade—COMPETE/QREN. MG Alves (SFRH/BPD/80451/2011) was funded by FCT. PF Oliveira was funded by FCT through FSE and POPH funds (Programa Ciência 2008).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, A.D., Alves, M.G., Bernardino, R.L. et al. Effect of white tea (Camellia sinensis (L.)) extract in the glycolytic profile of Sertoli cell. Eur J Nutr 53, 1383–1391 (2014). https://doi.org/10.1007/s00394-013-0640-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0640-5