Abstract
Background
Fish oil consisting of omega-3 polyunsaturated fatty acids (PUFA) seems to reduce the incidence of colon cancer. The effect of PUFAs on metastasis of colon carcinoma is still unclear.
Aim
The study was designed to examine the effects of a diet rich in omega-3-PUFAs on a model of colorectal metastasis.
Methods
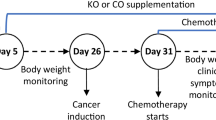
Thirthy animals (WAG/Rij) were randomly assigned to receive an omega-3 diet or a control diet to evaluate their effect on tumor growth. The target male rats (WAG/Rij) were fed a diet containing 15% omega-3-fatty acids three days before and 28 days after intervention and the control rats received 15% coconut oil at the same time points. CC 531 cells, a moderately differentiated colon adenocarcinoma, were injected into the spleen of each rat. After 28 days of diet, animals were sacrificed. The tumor growth was evaluated macroscopically and microscopically in liver tissue. The tissue was examined after immunostaining and the use of monoclonal antibodies.
Results
PUFAs decreased the index of tumor load from 1.54 in the controls to 0.79 in the treatment group (P = 0.036). While 69.2% of the control animals were tumor positive, only 21.4% of the target animals showed tumor after omega-3-fatty acid (P < 0.05).
Conclusion
We could show that omega-3-fatty acids may decrease malignant metastatic tumor growth in the liver.
Similar content being viewed by others
References
Stehr SN, Heller AR (2006) Omega-3 fatty acid effects on biochemical indices following cancer surgery. Clinica chimica acta. Int J Clin Chem 373:1–8
Bartsch H, Nair J, Owen R (1999) Dietary polyunsaturated fatty acids and cancer of the breast and colorectum: emerging evidence for their role as risk modi.ers. Carcinogenesis 20:2209–2218
Eastwood G (1996) Pharmacologic prevention of colonic neoplasms. E.ects of calcium, vitamins, omega fatty acids and nonsteroidal anti-in.ammatory drugs. Dig Dis Sci 14:119–128
Reddy B (1994) Chemoprevention of colon cancer by dietary fatty acids. Cancer Metastasis Rev 13:285–302
Begin ME, Ells G, Das UN (1986) Selected fatty acids as possible intermediates for selective cytotoxic activity of anticancer agents involving oxygen radicals. Anticancer Res 6:291–295
Ells GW, Chisholm KA, Simmons VA, Horrobin DF (1996) Vitamin E blocks the cytotoxic effect of gamma-linolenic acid when administered as late as the time of onset of cell death–insight into the mechanism of fatty acid induced cytotoxicity. Cancer Lett 98:207–211
Hansen PMB, McEntee MF, Chiu CH et al. (2000) Antagonism of arachidonic acid is linked to antitumorigenic effect of dietary eicosapentaenoic acid in APCMin/+ Mice. J Nutr 130:1153–1158
Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP (1998) Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res 58:3344–3352
German NS, Johanning GL (1997) Eicosapentaenoic acid and epidermal growth factor modulation of human breast cancer cell adhesion. Cancer Lett 118:95–100
Reddy B, Maruyama H (1986) E.ect of dietary .sh oil on azoxymethane induced colon carcinogenesis in male F344 rats. Cancer Res 46:3367–3370
Cannizzo F Jr., Broitman SA (1989) Postpromotional effects of dietary marine or safflower oils on large bowel or pulmonary implants of CT-26 in mice. Cancer Res 49:4289–4294
Sakaguchi M, Minoura T, Hiramatsu Y, Takada H, Yamamura M, Hioki K, Yamamoto M (1986) E.ects of dietary saturated and unsaturated fatty acids on fecal bile acids and colon carcinogenesis induced by azoxymethane in rats. Cancer Res 46:61–65
Latham P, Lund E, Johnson I (1999) Dietary n-3 PUFA increases the apoptotic response to 1,2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis 20:645–650
Latham P, Lund EK, Brown JC, Johnson IT (2001) Effects of cellular redox balance on induction of apoptosis by eicosapentaenoic acid in HT29 colorectal adenocarcinoma cells and rat colon in vivo. Gut 49:97–105
Iwamoto S, Senzaki H, Kiyozuka Y, Ogura E, Takada H, Hioki K, Tsubura A (1998) E.ects of fatty acids on liver metastasis of ACL-15 rat colon cancer cells. Nutr Cancer 31:143–150
Yang S, Morita I, Murota S (1998) Eicosapentaenoic acid attenuates vascular endothelial growth factor induced proliferation via inhibiting endothelial cells. J Cell Physiol 176:342–349
Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, Yazawa K, Araki E, Tsuda H (1997) Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br J Cancer 75:650–655
Suzuki I, Iigo M, Ishikawa C, Kuhara T, Asamoto M, Kunimoto T, Moore MA, Yazawa K, Araki E, Tsuda H (1997) Inhibitory effects of oleic and docosahexaenoic acids on lung metastasis by colon-carcinoma-26 cells are associated with reduced matrix metalloproteinase-2 and -9 activities. Int J Cancer 73:607–612
Griffini P, Fehres O, Klieverik L, Vogels IM, Tigchelaar W, Smorenburg SM, Van Noorden CJ (1998) Dietary omega-3 polyunsaturated fatty acids promote colon carcinoma metastasis in rat liver. Cancer Res 58:3312–3319
Bohle AS, Kalthoff H (1999) Molecular mechanisms of tumor metastasis and angiogenesis. Langenbecks Arch Surg 384:133–140
Haier J, Nasralla M, Nicolson GL (2000) Cell surface molecules and their prognostic values in assessing colorectal carcinomas. Ann Surg 231:11–24
Kinsella AR, Green B, Lepts GC, Hill CL, Bowie G, Taylor BA (1993) The role of the cell-cell adhesion molecule E-cadherin in large bowel tumour cell invasion and metastasis. Br J Cancer 67:904–909
Maurer CA, Friess H, Kretschmann B, Wildi S, Muller C, Graber H, Schilling M, Buchler MW (1998) Over-expression of ICAM-1, VCAM-1 and ELAM-1 might influence tumor progression in colorectal cancer. Int J Cancer 79:76–81
Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJ, Pals ST (1994) Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet 344:1470–1472
Jiang WG (1996) Cell adhesion molecules in the formation of liver metastasis. J Hepatobiliary Surg 5:375–382
Bouvy ND, Marquet RL, Jeekel H, Bonjer HJ (1996) Impact of gas (less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg 224:694–700; discussion 700–691
Danesch U, Weber PC, Sellmayer A (1996) Differential effects of n-6 and n-3 polyunsaturated fatty acids on cell growth and early gene expression in Swiss 3T3 fibroblasts. J Cell Physiol 168:618–624
Falconer JS, Ross JA, Fearon KC, Hawkins RA, O’Riordain MG, Carter DC (1994) Effect of eicosapentaenoic acid and other fatty acids on the growth in vitro of human pancreatic cancer cell lines. Br J Cancer 69:826–832
O’Connor TP, Roebuck BD, Peterson F, Campbell TC (1985) Effect of dietary intake of fish oil and fish protein on the development of L-azaserine-induced preneoplastic lesions in the rat pancreas. J Natl Cancer Inst 75:959–962
Rose DP, Connolly JM, Rayburn J (1995) Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst 87:587–592
Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K, Stillwell W, Zaloga GP, Siddiqui RA (2005) Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int J Cancer 117:340–348
Calder PC, Davis J, Yaqoob P, Pala H, Thies F, Newsholme EA (1998) Dietary fish oil suppresses human colon tumour growth in athymic mice. Clin Sci (Lond) 94:303–311
Anti M, Armelao F, Marra G et al. (1994) Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology 107:1709–1718
Hayashi Y, Fukushima S, Kishimoto S, Kawaguchi T, Numata M, Isoda Y, Hirano J, Nakano M (1992) Anticancer effects of free polyunsaturated fatty acids in an oily lymphographic agent following intrahepatic arterial administration to a rabbit bearing VX-2 tumor. Cancer Res 52:400–405
Lindner MA (1991) A fish oil diet inhibits colon cancer in mice. Nutr Cancer 15:1–11
Noguchi M, Minami M, Yagasaki R, Kinoshita K, Earashi M, Kitagawa H, Taniya T, Miyazaki I (1997) Chemoprevention of DMBA-induced mammary carcinogenesis in rats by low-dose EPA and DHA. Br J Cancer 75:348–353
Calviello G, Palozza P, Piccioni E, Maggiano N, Frattucci A, Franceschelli P, Bartoli GM (1998) Dietary supplementation with eicosapentaenoic and docosahexaenoic acid inhibits growth of Morris hepatocarcinoma 3924A in rats: effects on proliferation and apoptosis. Int J Cancer 75:699–705
Heukamp I, Kilian M, Gregor JI, Kiewert C, Schimke I, Kristiansen G, Walz MK, Jacobi CA, Wenger FA (2006) Impact of polyunsaturated fatty acids on hepato-pancreatic prostaglandin and leukotriene concentration in ductal pancreatic cancer – is there a correlation to tumour growth and liver metastasis? Prostag Leukotr Ess 74:223–233
Ambs S, Hussain S, Harris C (1997) Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J 11:443–448
Cooke J, Losordo D (2002) Nitric oxide and angiogenesis. Circulation 105:2133–2135
Lala P, Chakraborty C (2001) Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol 2:149–156
Weber C, Erl W, Pietsch A, Danesch U, Weber PC (1995) Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscl Throm Vas 15:622–628
De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA Jr, Libby P (1994) The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscl Thromb Vas 14:1829–1836
Martin D, Meckling-Gill KA (1996) Omega-3 polyunsaturated fatty acids increase purine but not pyrimidine transport in L1210 leukaemia cells. Biochem J 315 (Pt 1):329–333
Beck SA, Smith KL, Tisdale MJ (1991) Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res 51:6089–6093
Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Wang J, Luo C, Fujii T, Ichikawa H, Shirai T, Tokudome S (2003) Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett 193:17–24
Hofmanova J, Vaculova A, Lojek A, Kozubik A (2004) Interaction of polyunsaturated fatty acids and sodium butyrate during apoptosis in HT-29 human colon adenocarcinoma cells. Eur J Nutr:1–12
Acknowledgements
We thank the Else Kröner – Fresenius Foundation for the support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutt, C.N., Brinkmann, L., Mehrabi, A. et al. Dietary omega-3-polyunsaturated fatty acids prevent the development of metastases of colon carcinoma in rat liver. Eur J Nutr 46, 279–285 (2007). https://doi.org/10.1007/s00394-007-0662-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-007-0662-y