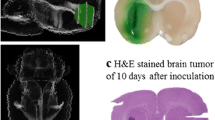
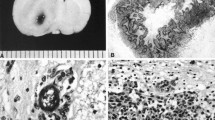
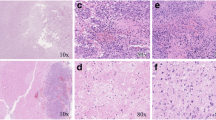
Abstract
Purpose
Local direct delivery of chemotherapeutic agents for the treatment of brain tumors is an area of focus in the development of new therapeutic paradigms. These techniques need improvement, especially in terms of drug retention in brain tissue.
Materials and methods
In this study, we used a rat glioma model to examine carboplatin distribution, as measured by platinum penetration, after delivery via interstitial continuous (i.c.) infusion. We also examined rat survival times in response to carboplatin and oxaliplatin. I.C. infusion, a modified version of convection-enhanced delivery (CED) for local drug delivery, uses low volume (1 μl per hour) continuous infusion directly into the tumor.
Results
I.C. infusion produced a nearly 360-fold higher concentration of platinum in tumor tissue and significantly prolonged rodent survival time compared to intraperitoneal (i.p.) infusion.
Conclusions
We showed i.c. infusion allows for circumvention of the blood–brain barrier, focused drug distribution, and sustained drug delivery. This method could be a promising strategy for treating brain tumors.





Similar content being viewed by others
References
Wolff JE, Trilling T, Mölenkamp G, Egeler RM, Jürgens H (1999) Chemosensitivity of glioma cells in vitro: a meta analysis. J Cancer Res Clin Oncol 125:481–486
Chamberlain MC (2006) Treatment options for glioblastoma. Neurosurg Focus 20:E19
Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Fiamengo SA, Neuwelt EA (1998) Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery 43:879–886
Groothuis DR (2000) The blood–brain and blood–tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol 2:45–59
Begley DJ (2004) Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 104:29–45
Brem H, Mahaley MS Jr, Vick NA, Black KL, Schold SC Jr, Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW, Kenealy JN (1991) Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg 74:441–446
Tomita T (1991) Interstitial chemotherapy for brain tumors: review. J Neurooncol 10:57–74
Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH (1995) Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg 82:1021–1029
Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH (1999) Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 90:315–320
Khan A, Jallo GI, Liu YJ, Carson BS Sr, Guarnieri M (2005) Infusion rates and drug distribution in brain tumor models in rats. J Neurosurg 102(Suppl.):53–58
Degen JW, Walbridge S, Vortmeyer AO, Oldfield EH, Lonser RR (2003) Safety and efficacy of convection-enhanced delivery of gemcitabine or carboplatin in a malignant glioma model in rats. J Neurosurg 99:893–898
Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, Bankiewicz KS (2004) Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res 64:6858–6862
Lévi F, Metzger G, Massari C, Milano G (2000) Oxaliplatin: pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet 38:1–21
Jerremalm E, Hedeland M, Wallin I, Bondesson U, Ehrsson H (2004) Oxaliplatin degradation in the presence of chloride: identification and cytotoxicity of the monochloro monooxalato complex. Pharm Res 21:891–894
Rochard E, Barthes D, Courtois P (1994) Stability and compatibility study of carboplatin with three portable infusion pump reservoirs. Int J Pharm 101:257–262
Kroin JS, Penn RD (1982) Intracerebral chemotherapy: chronic microinfusion of cisplatin. Neurosurgery 10:349–354
Chang MJ, Yu WD, Reyno LM, Modzelewski RA, Egorin MJ, Erkmen K, Vlock DR, Furmanski P, Johnson CS (1994) Potentiation by interleukin 1α of cisplatin and carboplatin antitumor activity: schedule-dependent and pharmacokinetic effects in the RIF-1 tumor model. Cancer Res 54:5380–5386
Erkmen K, Egorin MJ, Reyno LM, Morgan R Jr, Doroshow JH (1995) Effects of storage on the binding of carboplatin to plasma. Cancer Chemother Pharmacol 35:254–256
Butowski NA, Sneed PK, Chang SM (2006) Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin Oncol 24:1273–1280
Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S, Glioma Outcomes Investigators (2003) Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 99:467–473
Sugiyama S, Yamashita Y, Kikuchi T, Saito R, Kumabe T, Tominaga T (2007) Safety and efficacy of convection-enhanced delivery of ACNU, a hydrophilic nitrosourea, in intracranial brain tumor. J Neurooncol 82:41–47
Doz F, Berens ME, Dougherty DV, Rosenblum ML (1991) Comparison of the cytotoxic activities of cisplatin and carboplatin against glioma cell lines at pharmacologically relevant drug exposures. J Neurooncol 11:27–35
Wang PP, Frazier J, Brem H (2002) Local drug delivery to the brain. Adv Drug Deliv Rev 54:987–1013
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436
Chen W, Lu DR (1999) Carboplatin-loaded PLGA microspheres for intracerebral injection: formulation and characterization. J Microencapsul 16:551–563
Carson BS Sr, Wu Q, Tyler B, Sukav L, Raychaudhuri R, DiMeco F, Clatterbuck RE, Olivi A, Guarnieri M (2002) New approach to tumor therapy for inoperable areas of the brain: chronic intraparenchymal drug delivery. J Neurooncol 60:151–158
Friesen C, Herr I, Krammer PH, Debatin KM (1996) Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med 2:574–577
Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A (1995) Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1:129–134
Acknowledgments
This project was supported by the Rory David Deutsch Foundation, the Neuro-Oncology Research Foundation of Children’s Memorial Hospital, Chicago, Illinois and the Dr. Ralph and Marian C. Falk Medical Research Trust, Chicago, Illinois. We acknowledge expert technical assistance provided by Christopher D. McCabe, M.S. and Julianne Holleran (Medicine and Pharmacology, University of Pittsburgh Cancer Institute, Pittsburgh), The Department of Pathology Laboratory and Medicine, Children’s Memorial Hospital, Chicago, Illinois, and the editorial assistance of Rishi Lulla, M.D. and Ms. Terrie Byrne.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tange, Y., Kondo, A., Egorin, M.J. et al. Interstitial continuous infusion therapy in a malignant glioma model in rats. Childs Nerv Syst 25, 655–662 (2009). https://doi.org/10.1007/s00381-008-0805-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-008-0805-3