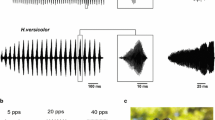
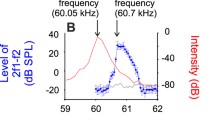
Abstract
We examined the mechanisms that underlie ‘band-suppression’ amplitude modulation selectivity in the auditory midbrain of anurans. Band-suppression neurons respond well to low (5–10 Hz) and high (>70 Hz) rates of sinusoidal amplitude modulation, but poorly, if at all, to intermediate rates. The effectiveness of slow rates of sinusoidal amplitude modulation is due to the long duration of individual ‘pulses’; short-duration pulses (<10 ms) failed to elicit spikes when presented at 5–10 pulses s−1. Each unit responded only after a threshold number of pulses (median=3, range=2–5) were delivered at an optimal rate. The salient stimulus feature was the number of consecutive interpulse intervals that were within a cell-specific tolerance. This interval-integrating process could be reset by a single long interval, even if preceded by a suprathreshold number of intervals. These findings indicate that band-suppression units are a subset of interval-integrating neurons. Band-suppression neurons differed from band-pass interval-integrating cells in having lower interval-number thresholds and broader interval tolerance. We suggest that these properties increase the probability of a postsynaptic spike, given a particular temporal pattern of afferent action potentials in response to long-duration pulses, i.e., predispose them to respond to slow rates of amplitude modulation. Modeling evidence is provided that supports this conclusion.







Similar content being viewed by others
Abbreviations
- AM:
-
amplitude modulation
- PRR:
-
pulse repetition rate
- SAM:
-
sinusoidal amplitude modulation
References
Albert M, Hose B, Langner G (1989) Modulation transfer functions in the auditory midbrain (MLD) of the guinea fowl (Mumida meleagris). In: Elsner N, Singer W (eds) Dynamics and plasticity in neuronal systems. Thieme, New York
Alder TB, Rose GJ (1998) Long-term temporal integration in the anuran auditory system. Nat Neurosci 1:519–523
Alder TB, Rose GJ (2000) Integration and recovery processes contribute to the temporal selectivity of neurons in the midbrain of the northern leopard frog, Rana pipiens. J Comp Physiol A 186:923–937
Brenowitz EA, Rose GJ (1994) Behavioural plasticity mediates aggression in choruses of the Pacific treefrog. Anim Behav 47:633–641
Capranica RR (1976) Morphology and physiology of the auditory system. In: Llinas R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 551–575
Capranica RR, Moffat AJM (1975) Selectivity of the peripheral auditory system of spadefoot toads (Scaphiopus couchi) to sounds of biological significance. J Comp Physiol A 100:231–249
Edwards CJ, Alder TB, Rose GJ (2002) Auditory midbrain neurons that count. Nat Neurosci 5:934–936
Falls JB (1963) Properties of bird song eliciting responses from territorial males. Proc Int Ornithol Congr 13:259–271
Gerhardt HC (1988). Acoustic properties used in call recognition by frogs and toads. In: Fritzch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 455–483
Huber F, Thorson J (1985) Cricket auditory communication. Sci Am 253(6, Dec):60–68
Olive JP, Greenwood A, Coleman J (1993). Acoustics of American English speech. A dynamic approach. Springer, Berlin Heidelberg New York
Rose G (1986) A temporal-processing mechanism for all species? Brain Behav Evol 28:134–144
Rose G, Brenowitz EA (1997) Plasticity of aggressive thresholds in Hyla regilla: discrete accommodation to encounter calls. Anim Behav 53:353–361
Rose GJ, Brenowitz EA (2002) Pacific treefrogs use temporal integration to differentiate advertisement from encounter calls. Anim Behav 63:1183–1190
Rose G, Capranica RR (1983) Temporal selectivity in the central auditory system of the leopard frog. Science 219:1087–1089
Rose G, Capranica RR (1984) Processing amplitude-modulated sounds by the auditory midbrain of two species of toads: matched temporal filters. J Comp Physiol A 154:211–219
Wilczynski W, Capranica RR (1984) The auditory system of anuran amphibians. Prog Neurobiol 22:1–38
Acknowledgements
The authors wish to thank Howard Gritton and Stephanie Plamondon for their help in acquiring and analyzing data. Anesthesia and surgical procedures were performed under the guidelines established by the Society for Neuroscience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, C.J., Rose, G.J. Interval-integration underlies amplitude modulation band-suppression selectivity in the anuran midbrain. J Comp Physiol A 189, 907–914 (2003). https://doi.org/10.1007/s00359-003-0467-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-003-0467-2