Abstract
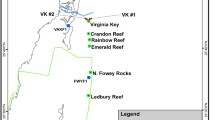
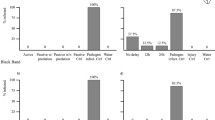
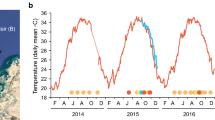
Anecdotal evidence collected since 2004 suggests that infections caused by ciliates in the genus Halofolliculina may be related to coral mortality in more than 25 scleractinian species in the Caribbean. However, the relationship between the presence of ciliates and coral mortality has not yet been firmly established. Field and laboratory manipulations were used to test if ciliate infections harm corals, if ciliates are able to infect healthy colonies, and if coral susceptibility to ciliate infection depends on temperature, depth, distance to an infected colony, and the presence of injuries. Ciliate infections were always characterized by a visually detectable front of ciliates located on recently exposed coral skeletons. These infections altered the normal structure of the colony by causing tissue mortality (0.8 ± 0.95 cm month−1, mean ± SD) and by delaying or preventing recovery from injuries. Under laboratory conditions, ciliates transmitted directly and horizontally from infected to healthy hosts, and coral susceptibility to ciliate infections increased with the presence of injuries. After invasion, the ciliate population grew, rapidly and after 8 d, produced tissue mortality on 32% of newly infected hosts. Thus, our results support the existence of a new Caribbean coral syndrome that is associated with tissue mortality, is infectious, and transmits directly and horizontally. Even though the role of ciliates in the development of lesions on coral tissues remains unclear, their presence is by far the most conspicuous sign of this syndrome; thus, we propose to name this condition Caribbean ciliate infection (CCI).







Similar content being viewed by others
References
Acosta A (2001) Disease in zoanthids: dynamics in space and time. Hydrobiologia 460:113–130
Aeby GS (1998) A digenean metacercaria from the reef coral, Porites compressa, experimentally identified as Podocotyloides stenometra. J Parasitol 84:1259–1261
Aeby GS (2002) Trade-offs for the butterflyfish, Chaetodon multicinctus, when feeding on coral prey infected with trematode metacercariae. Behav Ecol Sociobiol 52:158–163
Aeby GS, Santavy DL (2006) Factors affecting susceptibility of the coral Montastraea faveolata to black-band disease. Mar Ecol Prog Ser 318:103–110
Alker A, Smith G, Kim K (2001) Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean sea fan corals. Hydrobiologia 460:105–111
Antonius A (1985) Black band disease infection experiments on hexacorals and octocorals. Proc 5th Int Coral Reef Symp 6:155–160
Antonius AA, Lipscomb D (2001) First protozoan coral-killer identified in the Indo-Pacific. Atoll Res Bull 481:1–21
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Ben-Haim Y, Banim E, Kushmaro A, Loya Y, Rosenberg E (1999) Inhibition of photosynthesis and bleaching of zooxanthellae by the coral pathogen Vibrio shiloi. Appl Environ Microbiol 1:223–229
Borger JL, Steiner SCC (2005) The spatial and temporal dynamics of coral diseases in Dominica, West Indies. Bull Mar Sci 77:137–154
Boyett HV, Bourne DG, Willis BL (2007) Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar Biol 151:1711–1720
Bruckner AW, Bruckner RJ (1997) Outbreak of coral disease in Puerto Rico. Coral Reefs 16:260
Bruckner AW, Bruckner RJ (2006) Consequences of yellow band disease (YBD) on Montastraea annularis (species complex) populations on remote reefs off Mona Island, Puerto Rico. Dis Aquat Org 69:69–73
Bruckner A, Bruckner R, Williams EJ (1997) Spread of a black-band disease epizootic through the coral reef system in St. Ann’s Bay, Jamaica. Bull Mar Sci 61:918–928
Bruno JF, Laura E, Petes C, Harvell D, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases. Ecol Lett 6:1056–1061
Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol 5:1–8
Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Sicardi A, Sponga F (2000) A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol Lett 3:284–293
Cervino J, Goreau TJ, Negelkerken I, Smith GW, Hayes R (2001) Yellow band and dark spot syndromes in Caribbean corals: distributions, rate of spread, cytology, and effects on abundance and division rate of zooxanthellae. Hydrobiology 460:53–63
Cervino JM, Hayes RL, Polson SW, Polson SC, Goreau TJ, Martinez RJ, Smith GW (2004) Relationship of Vibrio species infection and elevated temperatures to Yellow Blotch/Band disease in Caribbean corals. Appl Environ Microbiol 70:6855–6864
Cook T, Folli M, Klinck J, Ford S, Miller J (1998) The relationship between increasing sea-surface temperature and the northward spread of Perkinsus marinus (Dermo) disease epizootics in oysters. Estuar Coast Shelf Sci 46:587–507
Cróquer A, Bastidas C, Lipscomb D (2006a) Folliculinid ciliates: a new threat to Caribbean corals? Dis Aquat Org 69:75–78
Cróquer A, Bastidas C, Lipscomp D, Rodríguez-Martínez RE, Jordan-Dahlgren E, Guzmán HM (2006b) First report of folliculinid ciliates affecting Caribbean scleractinian corals. Coral Reefs 25:187–191
Cooper W, Lirman D, Schmale M, Lipscomb D (2007) Consumption of coral spat by histophagic ciliates. Coral Reefs 26:249–250
De’ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192
Edmunds PJ (1991) Extent and effect of black band disease on a Caribbean reef. Coral Reefs 10:161–165
Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW (2002) Partitioning of bacterial communities between seawater and healthy black band diseased and dead coral surfaces. Appl Environ Microbiol 68:2214–2228
Guzmán HM, Guevara CA (1999) Arrecifes coralinos de Bocas del Toro, Panamá: III. Distribución, estructura, diversidad y estado de conservación de los arrecifes de las islasPastores, Cristóbal, Popa y Cayo Agua. Rev Biol Trop 47:1–9
Harvell CD, Kim K, Burlkholder JM, Colwell RR, Epstein PR, Grimes DJ, Homann EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR (1999) Emerging marine diseases, climate links and anthropogenic factors. Science 285:1505–1510
Kuta KG, Richardson LL (1996) Abundance and distribution of black band disease on coral reefs in northern Florida Keys. Coral Reefs 15:279–223
Kuta KG, Richardson LL (1997) Black band disease and the fate of diseases coral colonies in the Florida Keys. Proc 8th Coral Reef Symp 1:575–578
Kuta KG, Richardson LL (2002) Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs 21:393–398
Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol 346:36–44
Mullen KR, Peters EC, Harvell CD (2004) Coral resistance to disease. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer-Verlag, New York, pp 377–399
Nagelkerken I, Meesters EH, Bak RPM (1999) Depth-related variation in regeneration of artificial lesions in the Caribbean corals Porites astreoides and Stephanocoenia michelinii. J Exp Mar Biol Ecol 234:29–39
Nugues MM (2002) Impact of a coral disease outbreak on coral communities in St. Lucia: what and how much has been lost? Mar Ecol Prog Ser 229:61–71
Oren U, Rinkevich B, Loya Y (1997) Oriented intra-colonial transport of 14C labeled materials during coral regeneration. Mar Ecol Prog Ser 161:117–122
Page CA, Willis BL (2008) Epidemiology of skeletal eroding band on the Great Barrier Reef and the role of injury in the initiation of this widespread coral disease. Coral Reefs 27:257–272
Peters EC (1997) Diseases of coral-reef organisms. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 114–136
Richardson LL (1998a) Coral diseases: what is really known? Trends Ecol Evol 13:438–443
Richardson LL (1998b) Florida’s mystery coral killer identified. Nature 392:557–558
Richardson LL, Kuta KG (2003) Ecological physiology of the black band disease cyanobacterium Phormidium corallyticum. FEMS Microbiol Ecol 43:287–298
Riegl B (2002) Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE). Mar Biol 140:29–40
Sussman M, Loya Y, Fine M, Rosenberg E (2003) The marine fireworm Hermodice carunculata is a winter reservoir and spring–summer vector for the coral-bleaching pathogen. Appl Environ Microbiol 5:250–255
Sutherland KP, Porter JW, Torres C (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser 266:373–302
Voss JD, Richardson LL (2006) Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 25:569–573
Weil E (2004) Coral reef diseases in the wider Caribbean. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer-Verlag, New York, pp 35–68
Willis BL, Page CA, Dinsdale EA (2004) Coral disease in the Great Barrier Reef. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer-Verlag, New York, pp 69–104
Winkler R, Antonius A, Renegar DA (2004) The skeleton eroding band disease on coral reefs of Aqaba, Red Sea. Mar Ecol 25:129–144
Work TM, Aeby GS (2006) Systematically describing gross lesions in corals. Dis Aquat Org 70:155–160
Work TM, Richardson LL, Reynolds TL, Willis BL (2008) Biomedical and veterinary science can increase our understanding of coral disease. J Exp Mar Biol Ecol 362:63–70
Acknowledgments
The authors thank the Smithsonian Tropical Research Institute of Panama, which provided logistical support for this research, and Rachel Collins, director of Bocas Research Station. Special thanks go to Carlos Guevara for assistance in the field and to Plinio Gondola and Gabriel Jacome for logistical support. We thank Ruth Ramos and Denise Debrot whose comments helped to improve early drafts of this manuscript. Comments by two reviewers and a final proof reading by Frances Osborn are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr Michael Lesser
Rights and permissions
About this article
Cite this article
Rodríguez, S., Cróquer, A., Guzmán, H.M. et al. A mechanism of transmission and factors affecting coral susceptibility to Halofolliculina sp. infection. Coral Reefs 28, 67–77 (2009). https://doi.org/10.1007/s00338-008-0419-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-008-0419-y