Abstract
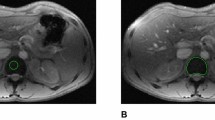
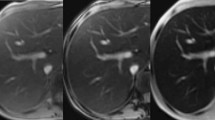
Thirty-seven patients with β-thalassemia major, including 14 adolescents (15.2 ± 3.0 years) and 23 adults (26.4 ± 6.9 years), were studied. T2 relaxation time (T2) of the liver, bone marrow, pancreas and pituitary gland was measured in a 1.5-Tesla magnetic resonance (MR) imager, using a multiecho spin-echo sequence (TR/TE 2,000/20, 40, 60, 80, 100, 120, 140, 160 ms). Pituitary gland height was evaluated in a midline sagittal scan of a spin-echo sequence (TR/TE, 500/20 ms). The T2 of the pituitary gland was higher in adolescents (59.4 ± 15 ms) than in adults (45.3 ± 10.4 ms), P < 0.05. The T2 of the pancreas was lower in adolescents (43.6 ± 10.3 ms) than in adults (54.4 ± 10.4 ms). No difference among groups was found in the T2 of the liver and bone marrow. There was no significant correlation of the T2 among the liver, pancreas, pituitary gland and bone marrow. There was no significant correlation between serum ferritin and T2 of the liver, pancreas and bone marrow. Pituitary T2 showed a significant correlation with pituitary gland height (adolescents: R = 0.63, adults: R = 0.62, P < 0.05) and serum ferritin (adolescents: R = −0.60, adults: R = −0.50, P < 0.05). In conclusion, iron overload evaluated by T2 is organ specific. After adolescence, age-related T2 changes are predominantly associated with pituitary siderosis and fatty degeneration of the pancreas. Pituitary size decreases with progressing siderosis.




Similar content being viewed by others
References
Rund D, Rachmilewitz E (2005) Beta-thalassemia. N Engl J Med 353:1135–1146
Forget BG (1993) The pathophysiology and molecular genetics of beta thalassemia. Mt Sinai J Med 60:95–103
Pootrakul P, Sirankapracha P, Hemsorach S et al (2000) A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood 96:2606–2612
Gabutti V, Piga A, Sacchetti L et al (1989) Quality of life and life expectancy in thalassemic patients with complications. Prog Clin Biol Res 309:35–41
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297
Gossuin Y, Muller RNGillis P (2004) Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification. NMR Biomed 17:427–432
Porter JB, Davis BA (2002) Monitoring chelation therapy to achieve optimal outcome in the treatment of thalassaemia. Best Pract Res Clin Haematol 15:329–368
St Pierre TG, Tran KC, Webb J et al (1991) Organ-specific crystalline structures of ferritin cores in beta-thalassemia/hemoglobin E. Biol Met 4:162–165
Gatter KC, Brown G, Trowbridge IS et al (1983) Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol 36:539–545
Atkin SL, Burnett HE, Green VL et al (1996) Expression of the transferrin receptor in human anterior pituitary adenomas is confined to gonadotrophinomas. Clin Endocrinol (Oxf) 44:467–471
Ernst O, Sergent G, Bonvarlet P et al (1997) Hepatic iron overload: diagnosis and quantification with MR imaging. AJR Am J Roentgenol 168:1205–1208
Midiri M, Lo Casto A, Sparacia G et al (1999) MR imaging of pancreatic changes in patients with transfusion-dependent beta-thalassemia major. AJR Am J Roentgenol 173:187–192
Christoforidis A, Haritandi A, Tsitouridis I et al (2006) Correlative study of iron accumulation in liver, myocardium, and pituitary assessed with MRI in young thalassemic patients. J Pediatr Hematol Oncol 28:311–315
Alexopoulou E, Stripeli F, Baras P et al (2006) R2 relaxometry with MRI for the quantification of tissue iron overload in beta-thalassemic patients. J Magn Reson Imaging 23:163–170
Argyropoulou MI, Metafratzi Z, Kiortsis DN et al (2000) T2 relaxation rate as an index of pituitary iron overload in patients with beta-thalassemia major. AJR Am J Roentgenol 175:1567–1569
Argyropoulou MI, Kiortsis DN, Efremidis SC (2003) MRI of the liver and the pituitary gland in patients with beta-thalassemia major: does hepatic siderosis predict pituitary iron deposition? Eur Radiol 13:12–16
St Pierre TG, Clark PR, Chua-anusorn W et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Metafratzi Z, Argyropoulou MI, Kiortsis DN et al (2001) T(2) relaxation rate of basal ganglia and cortex in patients with beta-thalassaemia major. Br J Radiol 74:407–410
Papakonstantinou O, Drakonaki EE, Maris T et al (2006) MR imaging of spleen in beta-thalassemia major. Abdom Imaging DOI 10.1007/s00261-006-9138-4
Papakonstantinou O, Ladis V, Kostaridou S et al (2006) The pancreas in beta-thalassemia major: MR imaging features and correlation with iron stores and glucose disturbances. Eur Radiol DOI 10.1007/s00330-006-0507-8
Drakonaki EE, Maris TG, Papadakis A et al (2006) Bone marrow changes in beta-thalassemia major: quantitative MR imaging findings and correlation with iron stores. Eur Radiol DOI 10.1007/s00330-006-0504-y
Argyropoulou MI, Kiortsis DN (2005) MRI of the hypothalamic-pituitary axis in children. Pediatr Radiol 35:1045–1055
Argyropoulou MI, Kiortsis DN, Metafratzi Z et al (2001) Pituitary gland height evaluated by MR in patients with beta-thalassemia major: a marker of pituitary gland function. Neuroradiology 43:1056–1058
Argyropoulou M, Perignon F, Brunelle F et al (1991) Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol 21:247–249
Olivieri NF (1999) The beta-thalassemias. N Engl J Med 341:99–109
Tiosano D, Hochberg Z (2001) Endocrine complications of thalassemia. J Endocrinol Invest 24:716–723
Bergeron C, Kovacs K (1978) Pituitary siderosis. A histologic, immunocytologic, and ultrastructural study. Am J Pathol 93:295–309
Rahier J, Loozen S, Goebbels RM et al (1987) The haemochromatotic human pancreas: a quantitative immunohistochemical and ultrastructural study. Diabetologia 30:5–12
Bronspiegel-Weintrob N, Olivieri NF, Tyler B et al (1990) Effect of age at the start of iron chelation therapy on gonadal function in beta-thalassemia major. N Engl J Med 323:713–719
Brittenham GM, Griffith PM, Nienhuis AW et al (1994) Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 331:567–573
Papakonstantinou OG, Maris TG, Kostaridou V et al (1995) Assessment of liver iron overload by T2-quantitative magnetic resonance imaging: correlation of T2-QMRI measurements with serum ferritin concentration and histologic grading of siderosis. Magn Reson Imaging 13:967–977
Gandon Y, Olivie D, Guyader D et al (2004) Non-invasive assessment of hepatic iron stores by MRI. Lancet 363:357–362
Papakonstantinou O, Kostaridou S, Maris T et al (1999) Quantification of liver iron overload by T2 quantitative magnetic resonance imaging in thalassemia: impact of chronic hepatitis C on measurements. J Pediatr Hematol Oncol 21:142–148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Argyropoulou, M.I., Kiortsis, D.N., Astrakas, L. et al. Liver, bone marrow, pancreas and pituitary gland iron overload in young and adult thalassemic patients: a T2 relaxometry study. Eur Radiol 17, 3025–3030 (2007). https://doi.org/10.1007/s00330-007-0683-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-007-0683-1