Abstract
Genetic engineering revolutionized the concept of traditional vaccines since subunit vaccines became reality. Additionally, over the past two decades plant-derived antigens have been studied as potential vaccines with several advantages, including low cost and convenient administration. More specifically, genetic fusions allowed the expression of fusion proteins carrying two or more components with the aim to elicit immune responses against different targets, including antigens from distinct pathogens or strains. This review aims to provide an update in the field of the production of plant-based vaccine, focusing on those approaches based on the production of chimeric proteins comprising antigens from human pathogens, emphasizing the case of cholera toxin/E. coli enterotoxin fusions, chimeric viruses like particles approaches as well as the possible use of adjuvant-producing plants as expression hosts. Challenges for the near future in this field are also discussed.
Similar content being viewed by others
Introduction
Jenner and Pasteur, the pioneers of vaccination in the 18th and 19th centuries, used attenuated or inactivated microorganisms to induce protective immunity to several pathogens. Their techniques, although crude, were successful to control many infectious diseases. However, in the recent decades, the emergence of new pathogens, such as HIV, has highlighted the need for continued development and improvement of vaccination strategies. Despite the efficiency of those early vaccines, there is concern over their safety, because they often have residual toxicity following inactivation and might contain toxic components, such as lipopolysaccharides (Ryan et al. 2001; Lavelle 2005).
This has changed the focus of vaccine development to safer although often less immunogenic, subunit vaccines composed of purified antigenic components of the microorganism. The biotechnological revolution made the large-scale production of recombinant proteins possible enabling the manufacture of these subunit vaccines. Molecular cloning techniques as well as improvements on gene synthesis have opened new doors in the rational design of subunit vaccines (Ryan et al. 2001; Lavelle 2005). In particular, chimeric proteins carrying epitopes from different pathogens, linkers, or adjuvant sequences offers not only increased immunogenicity of the recombinant antigen but also the possibility to elicit a broad cellular or humoral immune response (Berzofsky et al. 2001).
This can be obtained by selecting the epitopes capable of eliciting neutralizing antibodies. In addition, the epitopes must be conserved and preferably have to be linear and immunogenic per se. This approach also opened opportunities for the design of polyvalent vaccines trough the design and production of chimeras-carrying epitopes from different pathogens, strains or even different isolates. A number of models have demonstrated the feasibility to elicit broad immune responses using such a type of molecules (Berzofsky et al. 2001; Karlsson Hedestam et al. 2008). However, the formulations based on purified or recombinant subunit vaccines, are often poorly immunogenic and require adjuvants to help stimulate protective immunity.
Transgenic technologies have led to relevant application mainly in agriculture. Throughout the decade of the 90s, the concept of plant-based vaccines emerged as one of these applications. Plants would be used as production platform of well-characterized immunoprotective antigens and also as delivered vehicles. Plant-based vaccines attracted the attention of many research groups as this approach offers the advantages of mucosal immunization, along with other features, such as no requirement for fermentation, stability, low cost and a few side-effects; additionally, when the antigenic protein is produced in the edible parts of the plant, such as grain, fruit or even leaves, they may not require purification (Howard 2005). The first attempt to explore this biotechnological approach were directed to prove the principle that plants are capable to express functional full length antigens (Paul and Ma 2010). Veterinary field has also been exploring plant-based vaccine models against several pathogens, including the porcine transmissible gastroenteritis virus (Hammond and Nemchinov 2009), the highly pathogenic avian influenza virus (Kalthoff et al. 2010), additionally the case of an antibody fragment for passive immunity against gastrointestinal parasitic infections in chickens was reported (Zimmermann et al. 2009).
The production of chimeric proteins became the next logical focus for the development of plant-derived vaccines, with the advantages inherent to this approach. To date several reports have assessed the plant production of chimeric proteins as potential vaccines. This review describes the current status in the field, particularly those efforts to assess the expression of fusion proteins in order to produce vaccine candidates with broader immune protection in humans.
Multiepitopic protein design
The optimal vaccine against infections should be designed to selectively induce mainly protective immune responses and avoid undesirable ones. Such a vaccine must not therefore mimic the natural infection, but rather be engineered to contain only selected epitopes. The epitope selection must be based on their ability to induce protective immunity. Thus, in the rational design of artificial vaccines it is be necessary to identify appropriate epitopes that elicit protective helper and cytotoxic T cell and neutralizing antibody responses as well as to avoid epitopes that elicit harmful responses (Berzofsky et al. 2001; Karlsson Hedestam et al. 2008). Configuration of epitopes in a single polypeptide or protein is a critical factor for the immunogenic properties. This feature is almost only determined empirically although some softwares are available to identify potential epitopes as well as the protein structure of the desired chimeric protein.
The selection of linkers is particularly relevant in the design of functional chimeric proteins since they can play an important role on displaying specific epitopes in the overall structure of the fusion protein. Besides appropriate amino acid composition, the overall folding of the linker must be considered. According to Robinson and Sauer (1998) linkers have a significant effect on protein folding stability. It is undesirable to have a linker sequence with a high propensity to form α-helical or β-strand structures since they would limit the flexibility of the fusion protein and consequently affect its functional activity. The design of a linker sequence often requires careful consideration in order to avoid such secondary structural elements. Proline-containing sequences are, for example, often used as linkers to disrupt secondary structures allowing the display of epitopes fused at the end of the protein (Cardenas and Clements 1993). Many studies of linker peptides in various protein families have come to the conclusion that linkers lack regular secondary structure: they display varying degrees of flexibility to match their particular biological purpose and are rich in Ala, Pro and charged residues. Unfortunately, there are no reliable selection criteria available for using linker design. Most current linker design selection processes are still largely dependent on intuition. Although significant progress has been made in predicting secondary structures of proteins based on primary sequences, our understanding of sequence structure correlation is still limited (Crasto and Feng 2000).
Current status of chimeric protein approaches
Fusion proteins based on CT/LT
Cholera toxin (CT) and heat labile enterotoxin from ETEC (LT) are highly similar in both structure and function since both are ADP-ribosylating exotoxins (Spangler 1992). They consist of a receptor binding (B) oligomer formed by five identical B subunits (CTB/LTB) and an enzymatic subunit (CTA/LTA). CTB and LTB are highly immunogenic by oral route and lack toxicity (Field 1979). These features make CTB and LTB protein carriers attractive for the oral delivery of genetically attached antigens and epitopes (reviewed by Nashar et al. 1993). Using conventional microbial systems these proteins have been extensively used as carriers for unrelated antigens resulting in the induction of immune responses by mucosal routes (Reviewed by Nashar et al. 1993). This section summarized plant-based vaccination approaches based on the use of these carriers (Table 1).
Enteric diseases
Diarrhea caused by Vibrio cholera, enterotoxigenic Escherichia coli (ETEC) and rotavirus constitutes a major health issue worldwide, responsible for the highest levels of child morbidity and mortality in the developing countries (Schaetti 2009).
Yu and Langridge (2001) described an early attempt to express two chimeric proteins in potato, one comprising of CTB and the 22-amino acid immunodominant epitope of the murine rotavirus enterotoxin NSP4 while the second one consisted of the CTA2 and the ETEC fimbrial colonization factor CFA/I. The assembling of cholera holotoxin-like structures that retained the ability to bind the GM1 receptor was observed. However, cholera toxin neutralization assays were not performed, only protection was predicted based on the antibody titers. Later, this research group reported additional functional studies where the chimeric protein was able to induce antibodies capable to protect Caco2 cells against the binding of toxic E. coli, providing more evidence about the feasibility of plant-based multi-component vaccine protection against enterotoxigenic E. coli and rotavirus (Lee et al. 2004).
LTB has also been used as carrier for the heat stable toxin (ST) from ETEC. In a genetic fusion, ST was fused to the C-terminus of LTB (LTB::ST) and expressed in tobacco chloroplasts. Pentamers were detected by GM1-ELISA assay and anti-LTB humoral responses were triggered by feeding mice with tobacco leaves. Moreover, challenge with CT showed that mice immunized by the oral route were protected against CT activity (Rosales-Mendoza et al. 2009). A nuclear-encoded LTB:ST protein was also produced in tobacco, showing to be immunogenic at low doses which could be attributed to glycosilation and should be further analyzed (Rosales-Mendoza et al. 2011). Epitopes P4 and P6 from toxin co-regulated pilus subunit A (TCPA) from Vibrio cholerae were fused to CTB and expressed in tomato plants by Sharma et al. (2008b). Western blot and GM1-ELISA probed the synthesis and assembly of pentameric chimeric proteins in tomato fruit tissue. Immunization data was not yet presented, however. This approach has implications on the development of an efficient vaccine capable of inducing anti-toxin as well as anti-colonizing immunity against Vibrio cholerae infections.
Sharma et al. (2008a) reported the expression of the accessory colonization factor (acf) subunit A fused to CTB in tomato. Expression of the genetic fusion was confirmed, but only dimeric and trimeric forms were detected by Western blots under non-reducing conditions. The binding of CTB::VP7 fusion protein pentamers to GM1 ganglioside receptors was detected by enzyme-linked immunosorbent assay (ELISA). The feasibility of using these plants for the delivery of this rotavirus capsid protein antigen remains to be tested in animal models. Choi et al. (2005) obtained a genetic fusion comprising the coding regions for VP7 from rotavirus, the outer capsid protein of simian rotavirus SA11 and CTB. Potato transgenic plants were able to produce a pentameric CTB::VP7 fusion protein confirmed by GM1-ELISA. Although no immunization data was presented, these results demonstrated the ability of plant cells to produce the CTB-based chimeric proteins for rotavirus capsid antigens.
Human immunodeficiency virus
Infection with HIV, the causative agent of AIDS, is responsible for a large number of deaths every year worldwide and represents a significant threat to human health. Multiple HIV genes and proteins have been evaluated as candidate for HIV vaccines. Targets for vaccines include structural proteins (gag, env, gp120, gp41, and gp160), viral enzymes (pol), and regulatory proteins (nef, tat, rev and vpr). Matoba et al. (2009) explored the expression in Nicotiana benthamiana plants of chimeric protein CTB with aa 649-684 from the membrane proximal region (MPR) of the gp41 envelope protein, which is relevant since it plays a central role in several of the suggested mucosal HIV transmission pathways. The report provided evidence that GM1-binding pentameric CTB–MPR649–684 were assembled and triggered anti-MPR649–684 antibodies in a mouse model under a mucosal prime-systemic boost regimen.
The simian-human immunodeficiency virus (SHIV 89.6p) Tat regulatory element protein was fused to the c-terminus of the cholera toxin B subunit gene (ctxB-tat) and introduced into Solanum tuberosum cells. Oligomeric structures of pentamer size were detected in transformed tuber extracts by immunoblot analysis. The binding of CTB-Tat fusion protein pentamers to GM1 was proved by GM1-ELISA. The biological activity of this protein would indicate the feasibility of using this fusion against HIV-1 infection (Kim et al. 2004d).
CTB was fused to the simian immunodeficiency virus (SIVmac) Gag p27 capsid gene. The fusion CTB-Gag was produced in potato plants. Synthesis and assembly of the CTB-Gag fusion protein into oligomeric structures of pentamer size was confirmed by GM1-ELISA. Functionality of the carrier and adjuvant properties of CTB in a plant-based formulation subunit against SIV in macaques remains to be tested (Kim et al. 2004c).
The V3 loop has been produced as a fusion protein based on CTB using potato plants as host and the CTB-gp120 fusion protein was detected as oligomeric structures of pentamer size. GM1-ELISA analysis revealed expression levels ranging 0.002–0.004% TSP as well as GM1 binding ability (Kim et al. 2004b). The outer capsid protein of simian rotavirus SA11 had also been expressed through fusion with the carboxyl terminus of CTB. The transgenic Solanum tuberosum plants showed ability to synthesize and assemble the CTB::VP7 fusion protein into oligomers of pentameric size.
Tuberculosis
Components from M. tuberculosis, as the early secretory antigenic target, ESAT-6, and the antigen 85B (Ag85B) are antigens with vaccine potential. Rigano et al. (2004) reported the expression of Arabidopsis-derived tuberculosis (TB) antigen ESAT-6 fused to LTB. Plant material was fed to mice, inducing antigen-specific Th1 responses reporting for the first time an orally delivered, subunit, tuberculosis vaccine priming an antigen-specific response (Rigano et al. 2006).
Floss et al. (2010) have reported the production of a Ag85B:ESAT-6 fusion protein in tobacco plants by means of the elastin-like peptide (ELP) fusion strategy, which targets the protein with VPGVP motif derived from native mammalian elastins, facilitating purification by a procedure called inverse transition cycling. The level of production for this TBAg-ELP fusion protein reached up to 4% of the tobacco leaf total soluble proteins and it was able to trigger host antibodies and T cells recognizing mycobacterial Ag85B and probably ESAT-6 in mice and piglets immunized with purified TBAg-ELP or with crude tobacco leaf extracts.
Parasitic infections
The protective antigen As16 from the roundworm Ascaris suum has also been expressed as CTB fusion protein. This pathogen is a gastrointestinal nematode infecting both humans and animals, and the infection is endemic in many parts of the world (Crompton 2001). The plant model was rice and the transgenic seeds elicited in mice-specific serum antibody response when CT was used as adjuvant. Moreover, challenge experiment proved that immunized mice had a lower lung worm burden than control mice, suggesting that the rice-derived chimeric protein is a promising approach as an edible vaccine for controlling parasitic infections. Similar report was published for a CTB As14, however, no immunization experiment was presented (Nozoye et al. 2009).
Immunocontraceptive approach
Transgenic tomato plants were developed by Walmsley et al. (2003) to express a fusion protein consisting of the LTB and the ZP3 immunocontraceptive epitope. The fusion protein assembled pentamers, however, immunogenicity of the chimeric protein has not been reported so far.
Other target diseases
The 27 kDa domain I of anthrax lethal factor protein (LF), was expressed by Kim et al. (2004a) as fusion protein consisting of CTB to which carboxyl terminus fused to the antigen of interest. Potato plants (Solanum tuberosum) were transformed and immunoblot revealed the presence of the corresponding recombinant protein showing reactivity to both anti-CTB and anti-LF primary antibodies, thus verifying the synthesis and assembly of biologically active CTB-LF fusion protein oligomers. Using these plants to deliver adjuvant LF protein for CTB-mediated immunostimulation of mucosal immune responses against anthrax toxin remains to be tested (Kim et al. 2004a).
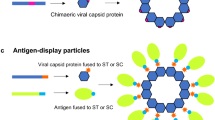
Fusion proteins based on VLPs
Another potential strategy to produce HIV vaccines is the use of VLP (virus-like particles) as fusion platforms for the mucosal delivery of heterologous antigens. The VLP have the ability to stimulate strong immune responses upon oral delivery, moreover, it has been proposed that the compact and highly ordered structures of VLP can provide resistance to digestive proteases (Huang et al. 2005). Among the main sequences used to produce VLP are the antigenic proteins for the hepatitis B virus (HBV). It was widely demonstrated that HBV VLP carrier spontaneously assembles in plant cells, giving VLPs with a preserved structure (Smith et al. 2003). See Table 2.
In 2007, a peptide containing eight epitopes of the proteins Gag and Pol from the HIV-1 (Michel et al. 2007) were produced through fusion with the surface protein of HBV in order to produce HIV-1::HBV VLPs. The recombinant protein was successfully expressed in Nicotiana tabacum and Arabidopisis thaliana. In both expression systems, HBV VLPs carrying the HIV-1 polypeptide on their surface were obtained, with comparable production levels (Greco et al. 2007).
Shchelkunov et al. (2006) described the development of tomato transgenic plants that produce a chimeric HBS::TBI containing the surface protein of hepatitis B virus (HBS) and Env and Gag epitopes of human immunodeficiency virus (TBI). Their studies demonstrate that oral administration of dried tomato tissue containing the chimeric HBS::TBI can elicit both serum and mucosal immune responses in mice. All of these results support the production of an efficient HIV::HBV bivalent vaccine against hepatitis B and the acquired immunodeficiency syndrome (AIDS), which could be particularly useful in the developing countries where the two diseases pose major public health issues.
Recently Martínez et al. (2010) produced in Nicotiana benthamiana plants, a fusion HB core protein, carrying a truncated version of the E-protein from Dengue virus. The antigenicity of the recombinant protein was proved trough the recognition by anti-E antibody, but its immunogenicity has not yet been assessed.
F1–V fusion proteins
Yersinia pestis, a Gram negative bacterium, is a dangerous pathogen that causes severe infections in humans: when inhaled, it can quickly induce fatal pneumonic plague; it still represents a serious public health threat in various parts of the world as well as a potential agent in bioterrorism (Prentice and Rahalison 2007; Titball and Leary 1998). Even though living and inactivated whole-cell vaccines are available for humans, serious drawbacks limit their use for prevention of outbreaks (Williamson et al. 1997). Thus, there is a need for stable, safe, and easily administered mucosal vaccines capable of eliciting effective protection against Y. pestis infections.
Two Y. pestis proteins, F1 and V, are known to be effective immunogens and have been proposed as candidates for a combined subunit vaccine against plague (Williamson et al. 1995). The fraction 1 capsular protein (F1) is a glycoprotein which is the major component of the Y. pestis polysaccharide capsule and is one of the primary antigens used in vaccines against plague because it has showed extremely high immunogenicity in both animals and humans. F1 is considered an important but not essential virulent factor unique to this pathogen (Andrews et al. 1996; Williamson et al. 1995; Welkos et al. 1995). The V antigen (LcrV) includes one subunit of the Y. pestis type III secretion system and is a second antigen commonly used for Y. pestis vaccines. It has been reported that V antigen has immunosuppressive potential by stimulating IL-10 secretion (Sing et al. 2002; Anderson et al. 1996).
A number of experimental vaccines produced in plants have been developed for plague infection (see Table 3). In the first approach, F1::V fusion antigens were expressed in tomato fruit. The immunogenicity of the recombinant protein was confirmed in mice that were subcutaneously primed with bacterially produced F1::V and then boosted orally with transgenic tomato fruit. It is noteworthy that, although nuclear transformation was employed, high concentrations of the fusion protein F1–V was achieved (4–10% of TSP) in green freeze-dried tomato fruit (Alvarez et al. 2006).
The same fusion protein has also been expressed in Nicotiana tabacum NT1 cells, Medicago sativa, carrot and lettuce, however, immune response in mice was only reported for the F1::V produced in carrot and lettuce (Alvarez and Cardineau 2010; Rosales-Mendoza et al. 2010a; Rosales-Mendoza et al. 2010b). In 2007, Mett et al. (2007) produced in Nicotiana benthamiana, F1 and V that were independently fused to a carrier molecule, LicKM, which is a thermostable enzyme lichenase from Clostridium thermocellum. The produced candidate vaccine conferred protection to monkeys against lethal challenge with Y. pestis.
A transplastomic approach was also pursued by Arlen et al. (2008) that revealed F1::V was expressed in tobacco plants; high levels of accumulation were reached (14% TSP) and it was immunogenic when administered orally to mice. Moreover, this plant-derived oral vaccine protected mice from live Y. pestis challenge. Another interesting approach was reported by Chichester et al. (2009). The F1::V fusion linked to the carrier molecule LicKM expressed in Nicotiana benthamiana was able to elicit systemic immune response protecting against Y. pestis challenge in macaques (Cynomolgus macaques). All of these results support the efficiency of plants as a production platform for plague vaccines.
HIV chimeric proteins
HIV antigens have been targeted in the form of a number of chimeric proteins (Table 4). The use of Ig fusion partners was proposed as a strategy for increasing the yield of a recombinant protein in plants (Obregon et al. 2006). HIV p24 antigen was expressed as a genetic fusion with the α-2 and α-3 constant region sequences from human Ig-chain in tobacco leaves. Immunological studies showed that the p24::IgA fusion protein was immunogenic in mice, suggesting a first step in the development of a new strategy for HIV vaccine at low cost and high yield. On the other hand, using a transplastomic approach, Zhou et al. (2008) reported the expression of two candidate HIV-1 antigens, p24 and Nef. This fusion protein was successfully expressed at high levels in plastids of tobacco and tomato. However, it has yet to be demonstrated that the chloroplast-derived HIV-1 antigens are able to induce neutralizing antibodies against HIV strains.
Chimeras targeting tetanus, diphtheria and pertussis
Vaccination against tetanus, diphtheria and pertussis (DPT) has markedly reduced the number of cases and deaths produced by the exotoxins of Corynebacterium diphteriae, Bordetella pertussis and Clostridium tetani. The first approach against a DPT vaccine became available during the 1920s and was composed of diphtheria and tetanus toxoids and the whole cell pertussis, however, due to this composition, several complications of the disease and secondary effects were frequent. In 1991, less reactogenic acellular pertussis vaccine (DTaP) was licensed (CDC 1992). Prompted by the local adverse reactions and reduced acceptance of the DTaP vaccine in adults, a simplified vaccine against these bacterial childhood diseases was formulated. It consists of a chimeric protein named sDPT made of two immunodominant protective epitopes from each one of the following: tetanus toxin heavy chain from C. tetani, the pertussis toxin subunit 1 of B., pertussis and diphtheria toxin subunit A of C. diphteriae (Soria-Guerra et al. 2007). This fusion protein was expressed in tomato plants by Agrobacterium mediated transformation as a first step to assess the functionality. Two adjuvant coding sequences from tetanus toxin and the endoplasmic retention signal SEKDEL were also included. Although the expression of the polypeptide was active in vitro, the expression levels in tomato plants reached 0.01% TSP (Soria-Guerra et al. 2007). Despite this relative low protein accumulation, a specific antibody response against the targetted antigens were detected in serum, gut, and lung samples from mice dosed orally with transgenic tomatoes (Soria-Guerra et al. 2011).
In order to enhance these expression levels, a transplastomic approach was conducted. A synthetic gene encoding for the DPT polypeptide was re-designed in order to optimize its expression in tobacco chloroplasts. Using biolistic-mediated transformation, several transformants were confirmed in the T1 progeny. The DPT protein content in tobacco leaves was 0.8% of TSP, which was approximately 110 times higher than that detected in tomato plants. Furthermore, it was showed that this candidate plant-based vaccine was immunogenic in mice by the oral route (Soria-Guerra et al. 2009). Although these results demonstrate the feasibility of plants as a platform for the generation of a recombinant subunit DTP vaccine, it is still necessary to perform toxin neutralization assays as well as challenges with each of the exotoxin in order to prove if protective immunity can be achieved.
Other potential dual vaccines
Studies on a prospective candidate for anthrax vaccination have also been conducted. In 2010, Andrianov et al. reported the production of chimeric recombinant protein (immunoadhesin) made of functional anthrax toxin receptor (ATR) domain fused with the human immunoglobulin Fc fragment (pATR-Fc). Previous works showed that the fusion of heterologous peptides with Fc provides better yields, stability, and facilitates purification process in plants (Obregon et al. 2006; Ashkenazi and Chamow 1997). It was observed that the recombinant protein protected J774A1 macrophage cells against the anthrax toxin, which suggests that plant-derived pATR-Fc antibody-like protein is suitable for post-infection therapy of anthrax. Likewise, a fusion protein comprising the epitope from domain-4 of the anthrax protective antigen (PA-D4) and the hepatitis B core antigen (HBcAg) was expressed in Nicotiana tabacum leaves. The recombinant protein was able to induce a systemic immune response; nevertheless, assembling of virus-like particles (VLP) was not observed (Bandurska et al. 2008). Among carrier proteins used in this field, elastin-like peptides (ELP) and a thermostable enzyme lichenase from Clostridium thermocellum (LicKM) yield promising results. Previous works have showed that ELP-fusions enhance the expression of many recombinant proteins and also have the advantage of an easy and inexpensive purification procedure due to the property of a thermally responsive reversible phase transition which avoids the cost-intensive affinity chromatography (Floss et al. 2009; Patel et al. 2007; Scheller et al. 2004).
The ELP plant-based expression strategy was successfully employed by Floss et al. (2010) in an approach to develop a subunit vaccine candidate against tuberculosis (TB). They produced the mycobacterial antigens Ag85B/ESAT- 6 (TBAg) as an ELP fusion in tobacco plants. The immunogenicity and safety of the plant expressed TBAg were confirmed in both mouse and piglet models. A vaccine against this disease would have an enormous impact on developing countries. In the same way, the E7 oncoprotein from Human Papilloma Virus (HPV) was fused to LicKM (Massa et al. 2007) with the aim of developing a candidate for cancer vaccine. The recombinant protein was expressed in N. benthamiana leaves and the immunogenicity and prophylactic potential of plant-produced LicKM::E7 was tested in mice. The reported results support the concept of producing an anti-tumor vaccine in plants.
In order to study the potential of plant expression systems to produce a candidate for subunit vaccine against dengue virus, the critical domain III serotype 2 of Dengue virus (DV2d3) was genetically fused to hepatitis B core antigen (HBcore) and expressed in N. benthamiana plants using the Magnifection system. This approach allows very fast production and high recombinant protein expression levels (Gleba et al. 2005a). The fusion DV2d3::HB core was produced at levels around 7% of TSP; furthermore, the protein was able to self-assembly into virus like-particles (VLPs), however, the immunological properties of the recombinant protein have yet to be analyzed (Martínez et al. 2010).
Another approach to produce a novel hepatitis B vaccine in plants was reported by Qian et al. (2008); they demonstrated that rice seeds are a suitable system for the production of the recombinant protein, containing the hepatocyte receptor-binding presurface 1 region (preS1) fused to major HBV surface protein (HBsA). They also demonstrated that the recombinant HBsA::preS1 produced in the transgenic seeds can assemble into VLP structures and induce immunological response in mice, thus implying that this rice-derived protein might have potential as a novel, oral, HBV candidate vaccine.
Immunological considerations
The most successful strategies for vaccination have been to mimic as closely as possible natural infection, therefore live-attenuated virus vaccines have been widely used. However, such strategy does not function for infections to which natural infection does not lead to long term immunity. Examples of this are parasitic and viral pathogens in which new infections are not prevented by the previous ones.
Most infectious agents enter the body at the mucosal surfaces and mucosal immune responses function as a first line of defense. Protective mucosal immune responses are most effectively induced by mucosal immunization through oral, nasal, rectal, or vaginal routes, but the vast majority of vaccines in use today are administered by intramuscular or subcutaneous injection, which are generally poor inducers of mucosal immunity (Ryan et al. 2001).
Mucosal vaccines that are given orally or deposited directly on the mucosal surfaces face the hosts’ defenses as do microbial pathogens: they are diluted in mucosal secretions, captured in mucus gels, attacked by proteases and nucleases, and excluded by epithelial barriers. Thus, relatively large dose of antigens are required and it is impossible to determine exactly what fraction crosses the mucosa. Most soluble non-adherent antigens are taken up in the intestine at low levels and such antigens generally induce tolerance. Thus, several vaccine formulations and delivery strategies have been used to address these challenges and have been revised extensively (Lavelle 2005).
Mucosal vaccines must be particulate or live-vectored, and include immunogenic-conserved forms. These formulations are likely to be most effective when they mimic successful mucosal pathogens in key aspects, efficiently stimulate innate responses, and evoke adaptative responses that are appropriate for the target pathogen. They are ideally multimeric and/or particulate, adhere to mucosal surfaces or even better, adhere to M cells (Ryan et al. 2001; Lavelle 2005).
High doses of antigen are required to induce protective immune responses by the oral route; this requires an efficient production platform. The initial attempts of using plants as platform production applied conventional nuclear transformation approaches, achieving low levels of expression which limited the dose that could be administered by the oral route. This report where a low level of antigen accumulation was observed followed standard strategies including the use of vectors carrying the gene of interest under the control of a strong promoter such as CaMV35S which is transferred to the plant’s genome via Agrobacterium (Mason et al. 1992).
Several approaches had been used to increase the protein accumulation in plant tissues. Among these, the optimization of synthetic genes according to codon usage for plants showed to enhance protein levels. The compartmentalization of the target protein through the use of retention signals, such as SEKDEL which direct the protein to endoplasmic reticulum, had a positive effect on the accumulation (Haq et al. 1995).
Later, transplastomic approaches were adopted as a promising strategy that allowed obtaining very high yields giving typical accumulation levels above 10% TSP (Cardi et al. 2010). Promising results have also been obtained by transient expression systems such as Magnifection which combines the viral replication mechanism with the ability of A. tumefaciens to transfer DNA to plant cells. These approaches seem to offer the best platform to provide the dose requirements for oral immunization. However, purification steps are necessary in order to rescue the protein of interest to be used for immunization (Gleba et al. 2005, 2007; Santi et al. 2008).
Seed-based expression through conventional nuclear transformation also seems to be a competitive platform since a large volume of recombinant proteins can be produced with increased content by the use of seed-specific promoters as well as localization signals. Additionally, the small size of most seeds allows for the recombinant protein to reach a relatively small volume and constitute a compact biomass in a stable environment being protected from degradation (Zimmermann et al. 2009; Stoger et al. 2005). This system also allows decoupling production of extraction and purification processes due to the storage properties of seeds. Certain seeds also have specific advantages, such as the presence of oil bodies, which can be exploited during the initial purification stages (Stoger et al. 2005). Besides promoters and enhancers, which regulate transcript abundance, subcellular trafficking and targeting of the desired proteins play a crucial role in their accumulation at high levels (Boothe et al. 2010).
Adjuvants
Adjuvants (from “adjuvare”: “to help”) are compounds that enhance the immunogenicity of vaccine antigens. A recent data suggest that most, if not all, adjuvants enhance T and B cell responses by engaging components of the innate immune system. Benefits of the adjuvant effects include the following: (1) an increase in the response to a vaccine in the general population, (2) an increase of seroconversion rates in populations with reduced responsiveness because of age, (3) the possibility to use smaller doses, (4) the reduction in the number of doses and (5) guiding the type of adaptive response to produce the most effective forms of immunity for each specific pathogen (review in Coffman et al. 2010).
Despite an explosion of knowledge regarding immune function over the recent decades, we remain almost totally reliant for human adjuvants on aluminum-based compounds whose activity was first discovered over 80 years ago. Recent advances in vaccine development and, in particular, the increasing use of recombinant subunit and synthetic vaccines makes the need for improved adjuvants much greater. There is a continuing major need for safe and non-toxic adjuvants, particularly for those capable of strongly boosting cellular immune responses. Despite many advances in immunology, adjuvant development is a priority goal in vaccinology.
A number of carrier proteins have adjuvant effects when epitopes are chemically linked or genetically fused. Besides LT or CT or their B subunits, recently described potential carrier proteins remain to be extensively explored in plant systems. Requirements for efficient carrier proteins are high resistance to low pH and intestinal proteases, and potent immunogenicity by the oral route. For example, the protoxin or toxin Cry1Ac from Bacillus thurigiensis might lead to new chimeric proteins to assess the ability to carry unrelated antigens eliciting strong mucosal immune responses (Vázquez et al. 1999; Vázquez-Padrón et al. 1999; Guerrero and Moreno-Fierros 2007).
Plant-derived adjuvants
The adjuvant activity of plant-derived compounds is particularly important in the development of mucosal vaccines. Since pathogens often enter mainly through the mucosal surfaces, new vaccines that elicit protective immunity by means of mucosal administration will be critical. Oral vaccine using plants offer the development of combinatorial approaches where plant producing endogenous adjuvants can be used to produce the recombinant antigen of interest, thus allowing the co-administration of both components, resulting in an enhanced and low cost formulation. This approach would be especially relevant for poor immunogenic proteins as well as for antigens with a low degree of protection against the pathogen.
Plants can produce a wide variety of compounds having adjuvant activity including polysaccharides, saponins, flavonoids, alkaloids and phenolics. Adjuvant activity has been displayed by many plant-derived compounds (reviewed by Licciardi and Underwood 2010).
It has been reported that the oral administration of phenolic-rich extracts of Mangifera indica increases 20 fold the systemic anti-SRBC HA response in mice (Makare et al. 2001). In a study by Nagai and Yamada (1994), oral adjuvanticity was observed by using extracts from the Sho-seiryu-to, a Japanese plant-derived medicine which contains the bioactive compound pinellic acid. This effect consisted of enhanced serum anti-influenza HI responses in mice. Moreover, increased anti-influenza virus IgA titres were also higher in the bronchoalveolar lavage fluid (BALF) and nasal wash (Nagai and Yamada 1994, 1998; Nagai et al. 1996).
Saponins from P. tenuifolia have also shown to have adjuvant activity producing an increase in serum anti-influenza titres in mice immunized by the in route (Nagai et al. 2001). This effect was attributed to enhanced APC activity and less antigen degradation due to the vaccine formulation leading to sustained exposure to the immune system. It could be proposed that such activity might ultimately provide heightened and prolonged T and/or B-lymphocyte activation resulting in the reported increased antibody response detected.
Similarly, the anti-influenza IgG response of mice orally administered with Pinellia ternata-derived extracts was elevated above control mice (Nagai et al. 2002). It was reported that this effect is attributed to pinellic acid, since oral administration of this compound showed the same enhancement on the IgG responses, suggesting that both systemic and mucosal responses can be modulated by this plant-derived adjuvant. These properties of P. ternata seem to be associated with indirect activation of B-lymphocytes possibly via APC stimulation similar to the Polygala plant-derived compounds. More recently, Quan et al. (2007) reported that the intranasal co-administration of inactivated influenza virus A and P. ginseng on days 0 and 14 significantly enhanced the humoral IgG response to influenza virus, effect likely associated to its high saponin content. Increased lung IgA was also observed 15 days after challenge with influenza virus, this evidence suggests a P. ginseng mediated modulation on systemic and mucosal immunity.
Saponins from Chenopodium quinoa have shown an adjuvant effect on the cholera toxin (CTX) immunization by the intranasal route (Estrada et al. 1998) which was deducted by increased intestinal serum and lung anti-CTX IgG levels suggesting that these saponins may directly activate the mucosal associated lymphoid system (MALT).
The reported immunomodulatory properties of Echinacea have been examined in a number of studies. Echinacea is one of the most widely used medicinal plants. A study by Rehman et al. (1999) demonstrated that the oral treatment of rats with an Echinacea angustifolia root extract containing a rich source of phenolic compounds (Kuzel et al. 2009) significantly elevated the IgG response to keyhole limpet hemocyanin (KLH) after three immunizations compared to control rats. Similarly, mice orally administered with a combination of E. purpurea and Echinacea pallidae root extracts increased the anti-SRBC. It was reported that the bioactive properties can differ widely between the various Echinacea species, therefore future studies should be based on standardized and well-characterized preparations. Also confirmation of its immune enhancing properties remains to be fully validated in human clinical trials. The vast number of studies using various forms of Echinacea and purified compounds make difficult to state conclusive arguments for their use as adjuvants in human vaccines (Pleschka et al. 2009).
Such wide number of plant-derived bioactive molecules with adjuvant properties constitutes promising candidates to be assessed on plant-derived vaccines formulations by co-administration or using the plants able to produce these compounds as expression hosts for the antigen of interest. This trend could lead to new generation of plant-derived vaccines.
Cytokines
Interleukines can play a role as adjuvants influencing the Th1/Th2 balance in the elicited immune response. Some interleukins have been expressed in plants showing to retain their functional properties. This suggests that co-expression of these molecules along with the antigen of interest could lead to adjuvant formulations with the ability of inducing strong immune responses, especially toward predominant Th1 responses which are rarely elicited by soluble subunit vaccines administered by the oral route.
Some cytokines have the potential to serve as adjuvants in the mucosal administration of immunogens. For example, Interleukin 12 (IL-12) is a key cytokine produced by a variety of APCs and promotes cell-mediated Th1 responses, therefore having implications in cancer immunotherapy, treatment of infectious diseases and as adjuvant in vaccine formulations. Mouse IL-12 expression in tomato plants was reported by Gutiérrez-Ortega et al. (2005). This recombinant IL-12 showed to be functional in vitro, determined by interferon-gamma (IFN-gamma) secretion by T cell cultures (Gutiérrez-Ortega et al. 2005). In a later report, functional studies showed that crude extracts of IL-12 administered by the intratracheal route have a specific biological activity reflected as IFN-gamma expression in lungs (Sánchez-Hernández et al. 2010). The expression of a single-chain human interleukin-12 in transgenic tobacco plants has also been reported. The biological activity was proved by IFN-gamma production assays which revealed comparable levels with respect to the commercially available recombinant IL-12 (Gutiérrez-Ortega et al. 2004). Another human interleukin, hIL-18, was successfully expressed in tobacco confirming its activity by the induction of IFN-gamma on J6-1 cells by plant extracts (Zhang et al. 2003).
Although IL-10 is an anti-inflammatory cytokine, it constitutes another example of a biologically active cytokine produced in plants when administered orally. In 2007, the production of human IL-10 in a low-alkaloid tobacco was described. Its evaluation as contra-inflammatory cytokine reflected a diminution in the severity of colitis in an inflammatory bowel disease animal model (Menassa et al. 2007; Conley et al. 2010). An approach related to cytokine’s action was reported by Conrad et al. (2011) whose study consisted in the production of an anti-human TNF single-domain monoclonal antibody (VHH), a major pro-inflammatory cytokine. The aim was to evaluate its potential as TNF antagonist which may have implications on the treatment of inflammatory diseases such as rheumatoid arthritis, Crohn’s disease and psoriasis. The fusion protein comprised VHH linked to an elastin-like polypeptide (ELP) and it was produced in tobacco plants. The heterologous protein showed to be biologically active in vivo and it was effective in preventing death caused by septic shock in mice.
These examples opened the doors to the production of functional cytokines that might be expressed in plants which in turn may supplement the plant material containing the antigen of interest. Another approach would be co-expressing both the cytokine and the antigen in the same plant, leading to a single source of the desired formulation. However, expression levels should be regulated to attain the appropriate doses of both components. Studies still need to be carried out to assess the feasibility of these approaches.
Concluding remarks
Plant-based vaccines targeting different diseases through the use of chimeric proteins as immunogens have been of great interest to vaccine developers. Progress over the past decade in the design and evaluation of new broad-protective proteins has demonstrated the feasibility of this technology. However, it is clear that there are a number of new approaches that might improve these candidate vaccines. Although the expression of proteins with the expected activity evaluated in vitro has attracted most of the attention and has been extensively explored, it is clear that strategies for formulation, delivery and evaluation of immunogenicity and even efficiency would profoundly influence the impact of this approach on the vaccine development field. Enhancing specific types of immune responses and generating the multifaceted immune responses that may be needed for challenging diseases is a priority goal.
Along the path of development of new vaccines lies the necessity to a significant investment in manufacturing plants, in human trials which are of great importance and in regulatory aspects before its potential can be exploited. The concept has had many proofs of its efficiency, nonetheless regulatory hurdles will need to be overcome, and industry and public acceptance of the technology are important in order to establish successful products.
References
Alvarez ML, Cardineau GA (2010) Prevention of bubonic and pneumonic plague using plant derived vaccines. Biotechnol Adv 28:184–196
Alvarez ML, Pinyerd HL, Crisantes JD, Rigano M, Pinkhasov J, Walmsley AM, Mason HS, Cardineau GA (2006) Plant-made subunit vaccine against pneumonic and bubonic plague is orally immunogenic in mice. Vaccine 24:2477–2490
Anderson GW, Leary SE, Williamson DE, Titball RW, Welkos SL, Worsham PL, Friedlander AM (1996) Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect Immun 64:4580–4585
Andrews GP, Heath DG, Anderson GW, Welkos SL, Friedlander AM (1996) Fraction 1 capsular antigen purification from Yersinia pestis CO92 and from an E. coli recombinant strain and efficacy against lethal plague challenge. Infect Immun 64:2180–2187
Andrianov V, Brodzik R, Spitsin S, Bandurska K, McManus H, Koprowski H, Golovkin M (2010) Production of recombinant anthrax toxin receptor (ATR/CMG2) fused with human Fc in planta. Prot Expr Pur 70:158–162
Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H (2008) Effective plague vaccination via oral delivery of plant cells expressing F1–Vs in chloroplasts. Infect Immun 76:3640–3650
Ashkenazi A, Chamow S (1997) Immunoadhesins as research tools and therapeutic agents. Curr Opin Immunol 9:195–200
Bandurska K, Brodzik R, Spitsin S, Kohl T, Portocarrero C, Smirnov Y, Pogrebnyak N, Sirko A, Koprowski H, Golovkin M (2008) Plant- produced hepatitis B core protein chimera carrying anthrax protective antigen domain-4. Hybridoma 27:241–247
Berzofsky JA, Ahlers JD, Belyakov IM (2001) Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol 1:209–219
Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, Murray E, Morck D, Moloney MM (2010) Seed-based expression systems for plant molecular farming. Plant Biotechnol J 8:588–606
Cardenas L, Clements JD (1993) Development of mucosal protection against the heat stable enterotoxin (ST) of Escherichia coli by oral immunization with a genetic fusion delivered by a bacterial vector. Infect Immun 61:4629–4636
Cardi T, Lenzi P, Maliga P (2010) Chloroplasts as expression platforms for plant-produced vaccines. Expert Rev Vaccines 9:893–911
CDC (1992) Pertussis vaccination: acellular pertussis vaccine for the fourth and fifth doses of the DTP series update to supplementary ACIP statement. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 41 (No. RR-15):1–5
Chichester JA, Musiychuk K, Farrance CE, Mett V, Lyons J, Mett V, Yusibov V (2009) A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine 27:3471–3474
Choi NW, Estes MK, Langridge WH (2005) Synthesis and assembly of a cholera toxin B subunit-rotavirus VP7 fusion protein in transgenic potato. Mol Biotechnol 31:193–202
Coffman RL, Sher A, Seder RA (2010) Vaccine adjuvants: putting innate immunity to work. Immunity. doi:10.1016/j.immuni.2010.10.002
Conley AJ, Zhu H, Le LC, Jevnikar AM, Lee BH, Brandle JE, Menassa R (2010) Recombinant protein production in a variety of Nicotiana hosts: a comparative analysis. Plant Biotechnol J. doi:10.1111/j.1467-7652.2010.00563.x
Conrad U, Plagmann I, Malchow S, Sack M, Floss DM, Kruglov AA, Nedospasov SA, Rose-John S, Scheller J (2011) ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock. Plant Biotechnol J 9:22–31
Crasto CJ, Feng J (2000) LINKER: a program to generate linker sequences for fusion proteins. Protein Eng 13:309–312
Crompton DW (2001) Ascaris and ascariasis. Adv Parasitol 48:285–375
Davoodi-Semiromi A, Schreiber M, Nalapalli S, Verma D, Singh ND, Banks RK, Chakrabarti D, Daniell H (2010) Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J 8:223–242
Estrada A, Li B, Laarveld B (1998) Adjuvant action of Chenopodium quinoa saponins on the induction of antibody responses to intragastric and intranasal administered antigens in mice. Comp Immunol Microbiol Infect Dis 21:225–236
Field M (1979) Modes of action of enterotoxins from Vibrio cholerae and Escherichia coli. Rev Infect Dis 1:918–926
Floss DM, Schallau K, Rose-John S, Conrad U, Scheller J (2009) Elastin-like polypeptides revolutionize recombinant protein expression and their biomedical application. Trends Biotechnol 28:37–45
Floss DM, Mockey M, Zanello G, Brosson D, Diogon M, Frutos R, Bruel T, Rodrigues V, Garzon E, Chevaleyre C, Berri M, Salmon H, Conrad U, Dedieu L (2010) Expression and immunogenicity of the mycobacterial Ag85B/ESAT-6 antigens produced in transgenic plants by elastin-like peptide fusion strategy. J Biomed Biotechnol 2010:274346
Gleba Y, Klimyuk V, Marillonnet S (2005) Magnifection—a new platform for expressing recombinant vaccines in plants. Vaccine 23:2042–2048
Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18:134–141
Greco R, Michel M, Guetard D, Cervantes-Gonzalez M, Pelucchi N, Wain-Hobson S, Sala F, Sala M (2007) Production of recombinant HIV-1/HBV virus-like particles in Nicotiana tabacum and Arabidopsis thaliana plants for a bivalent plant-based vaccine. Vaccine 25:8228–8240
Guerrero GG, Moreno-Fierros L (2007) Carrier potential properties of Bacillus thuringiensis Cry1A toxins for a diphtheria toxin epitope. Scand J Immunol 66:610–618
Gutiérrez-Ortega A, Avila-Moreno F, Saucedo-Arias LJ, Sánchez-Torres C, Gómez-Lim MA (2004) Expression of a single-chain human interleukin-12 gene in transgenic tobacco plants and functional studies. Biotechnol Bioeng 85:734–740
Gutiérrez-Ortega A, Sandoval-Montes C, de Olivera-Flores TJ, Santos-Argumedo L, Gómez-Lim MA (2005) Expression of functional interleukin-12 from mouse in transgenic tomato plants. Transgenic Res 14:877–885
Hammond RW, Nemchinov LG (2009) Plant production of veterinary vaccines and therapeutics. Curr Top Microbiol Immunol 332:79–102
Haq TA, Mason HS, Clements JD, Arntzen CJ (1995) Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268:714–716
Howard JA (2005) Commercialization of biopharmaceutical and bioindustrial proteins from plants. Crop Sci 45:468–472
Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen CJ, Thanavala Y, Mason HS (2005) Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine 23:1851–1858
Kalthoff D, Giritch A, Geisler K, Bettmann U, Klimyuk V, Gleba Y, Hehnen HS, Beer M (2010) Immunization with plant-expressed hemagglutinin protects chickens from lethal highly pathogenic avian influenza virus H5N1 challenge infection. J Virol 84:12002–12010
Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT (2008) The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol 6:143–155
Kim TG, Galloway DR, Langridge WH (2004a) Synthesis and assembly of anthrax lethal factor-cholera toxin B-subunit fusion protein in transgenic potato. Mol Biotechnol 28:175–183
Kim TG, Gruber A, Langridge WH (2004b) HIV-1 gp120 V3 cholera toxin B subunit fusion gene expression in transgenic potato. Protein Expr Purif 37:196–202
Kim TG, Gruber A, Ruprecht RM, Langridge WH (2004c) Synthesis and assembly of SIVmac Gag p27 capsid protein cholera toxin B subunit fusion protein in transgenic potato. Mol Biotechnol 28:33–40
Kim TG, Ruprecht R, Langridge WH (2004d) Synthesis and assembly of a cholera toxin B subunit SHIV 89.6p Tat fusion protein in transgenic potato. Protein Expr Pur 35:313–319
Kuzel S, Vydra J, Triska J, Vrchotova N, Hruby M, Cigler P (2009) Elicitation of pharmacologically active substances in an intact medical plant. J Agric Food Chem 57:7907–7911
Lavelle EC (2005) Generation of improved mucosal vaccines by induction of innate immunity. Cell Mol Life Sci 62:2750–2770
Lee JY, Yu J, Henderson D, Langridge WH (2004) Plant-synthesized E. coli CFA/I fimbrial protein protects Caco-2 cells from bacterial attachment. Vaccine 23:222–231
Li D, O’Leary J, Huang Y, Huner NP, Jevnikar AM, Ma S (2006) Expression of cholera toxin B subunit and the B chain of human insulin as a fusion protein in transgenic tobacco plants. Plant Cell Rep 25:417–424
Licciardi PV, Underwood JR (2010) Plant-derived medicines: a novel class of immunological adjuvants. Int Immunopharmacol (in press)
Makare N, Bodhankar S, Rangari V (2001) Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol 78:133–137
Martínez CA, Topal E, Giulietti AM, Talou JR, Mason H (2010) Exploring different strategies to express Dengue virus envelope protein in a plant system. Biotechnol Lett 32:867–875
Mason HS, Lam DM, Arntzen CJ (1992) Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci USA 89:11745–11749
Massa S, Franconi R, Brandi R, Muller A, Mett V, Yusibov V, Venuti A (2010) Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine 25:3018–3021
Matoba N, Kajiura H, Cherni I, Doran JD, Bomsel M, Fujiyama K, Mor TS (2009) Biochemical and immunological characterization of the plant-derived candidate human immunodeficiency virus type 1 mucosal vaccine CTB–MPR. 649–684. Plant Biotech J 7:129–145
Matsumoto Y, Suzuki S, Nozoye T, Yamakawa T, Takashima Y, Arakawa T, Tsuji N, Takaiwa F, Hayashi Y (2009) Oral immunogenicity and protective efficacy in mice of transgenic rice plants producing a vaccine candidate antigen (As16) of Ascaris suum fused with cholera toxin B subunit. Transgenic Res 18:185–192
Menassa R, Du C, Yin ZQ, Ma S, Poussier P, Brandle J, Jevnikar AM (2007) Therapeutic effectiveness of orally administered transgenic low-alkaloid tobacco expressing human interleukin-10 in a mouse model of colitis. Plant Biotechnol J 5:50–59
Mett V, Lyons J, Musiychuk K, Chichester JA, Brasil T, Couch R, Sherwood R, Palmer GA, Streatfield SJ, Yusibov V (2007) A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine 25:3014–3017
Michel M, Lone YC, Centlivre M, Roux P, Wain-Hobson S, Sala M (2007) Optimisation of secretion of recombinant HBsAg virus-like particles: impact on the development of HIV-1/HBV bivalent vaccines. Vaccine 25:1901–1911
Nagai T, Yamada H (1994) In vivo anti-influenza virus activity of kampo (Japanese herbal) medicine “Sho-seiryu-to” and its mode of action. Int J Immunopharmacol 16:605–613
Nagai T, Yamada H (1998) In vivo anti-influenza virus activity of Kampo (Japanese herbal) medicine “Sho-seiryu-to”—stimulation of mucosal immune system and effect on allergic pulmonary inflammation model mice. Immunopharmacol Immunotoxicol 20:267–281
Nagai T, Urata M, Yamada H (1996) In vivo anti-influenza virus activity of Kampo (Japanese herbal) medicine “Sho-seiryu-to”—effects on aged mice, against subtypes of a viruses and B virus, and therapeutic effect. Immunopharmacol Immunotoxicol 18:193–208
Nagai T, Suzuki Y, Kiyohara H, Susa E, Kato T, Nagamine T, Hagiwara Y, Tamura S, Yabe T, Aizawa C, Yamada H (2001) Onjisaponins, from the root of Polygala tenuifolia Willdenow, as effective adjuvants for nasal influenza and diphtheria–pertussis–tetanus vaccines. Vaccine 19:4824–4834
Nagai T, Kiyohara H, Munakata K, Shirahata T, Sunazuka T, Harigaya Y, Yamada H (2002) Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int Immunopharmacol 2:1183–1193
Nashar TO, Amin T, Marcello A, Hirst TR (1993) Current progress in the development of the B subunits of cholera toxin and Escherichia coli heat-labile enterotoxin as carriers for the oral delivery of heterologous antigens and epitopes. Vaccine 11:235–240
Nozoye T, Takaiwa F, Tsuji N, Yamakawa T, Arakawa T, Hayashi Y, Matsumoto Y (2009) Production of Ascaris suum As14 protein and its fusion protein with cholera toxin B subunit in rice seeds. J Vet Med Sci 71:995–1000
Obregon P, Chargelegue D, Drake PM, Prada A, Nuttall J, Frigerio L, Ma JK (2006) HIV-1 p24–immunoglobulin fusion molecule: a new strategy for plant-based protein production. Plant Biotech J 4:195–207
Patel J, Zhu H, Menassa R, Gyenis L, Richman A, Brandle J (2007) Elastin-like polypeptide fusions enhance the accumulation of recombinant proteins in tobacco leaves. Transgenic Res 16:239–249
Paul M, Ma JK (2010) Plant-made immunogens and effective delivery strategies. Expert Rev Vaccines 9:821–833
Pleschka S, Stein M, Schoop R, Hudson JB (2009) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol J 6:197
Prentice MB, Rahalison L (2007) Plague. Lancet 369:1196–1207
Qian B, Shen H, Liang W, Guo X, Zhang Ch, Wang Y, Li G, Wu A, Cao K, Zhang D (2008) Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 17:621–631
Quan FS, Compans RW, Cho YK, Kang SM (2007) Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 25:272–282
Rehman J, Dillow JM, Carter SM, Chou J, Le B, Maisel AS (1999) Increased production of antigen-specific immunoglobulins G and M following in vivo treatment with the medicinal plants Echinacea angustifolia and Hydrastis canadensis. Immunol Lett 68:391–395
Rigano MM, Alvarez ML, Pinkhasov J, Jin Y, Sala F, Arntzen CJ, Walmsley AM (2004) Production of a fusion protein consisting of the enterotoxigenic Escherichia coli heat-labile toxin B subunit and a tuberculosis antigen in Arabidopsis thaliana. Plant Cell Rep 22:502–508
Rigano MM, Dreitz S, Kipnis AP, Izzo AA, Walmsley AM (2006) Oral immunogenicity of a plant-made, subunit, tuberculosis vaccine. Vaccine 24:691–695
Robinson CR, Sauer RT (1998) Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc Natl Acad Sci USA 95:5929–5934
Rosales-Mendoza S, Alpuche-Solís AG, Soria-Guerra RE, Moreno-Fierros L, Martínez-González L, Herrera-Díaz A, Korban SS (2009) Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J 57:45–54
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Martínez-González L, Alpuche-Solís AG, Korban SS (2010a) Expression of an immunogenic F1–V fusion protein in lettuce as a plant-based vaccine against plague. Planta 232:409–416
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Han Y, Alpuche-Solís AG, Korban SS (2010b) Transgenic carrot tap roots express the immunogenic F1–V fusion protein from Yersinia pestis are immunogenic in mice. J Plant Physiol 168:174–180
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Govea-Alonso DO, Herrera-Díaz A, Korban SS, Alpuche-Solís AG (2011) Immunogenicity of nuclear-encoded LTB:ST fusion protein from Escherichia coli expressed in tobacco plants. Plant Cell Rep. doi:10.1007/s00299-011-1023-0
Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H (2007) Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts—oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J 5:495–510
Ryan EJ, Daly LM, Mills KH (2001) Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol 19:293–304
Sánchez-Hernández C, Gutiérrez-Ortega A, Aguilar-León D, Hernández-Pando R, Gómez-Lim M, Gómez-García B (2010) In vivo activity of plant-based interleukin-12 in the lung of Balb/c mouse. BMC Res Notes 3:151
Santi L, Batchelor L, Huang Z, Hjelm B, Kilbourne J, Arntzen CJ, Chen Q, Mason HS (2008) An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine 26:1846–1854
Schaetti C (2009) Vaccines for enteric diseases: update on recent developments. Expert Rev Vaccines 8:1653–1655
Scheller J, Henggeler D, Viviani A, Conrad U (2004) Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res 13:51–57
Sharma MK, Jani D, Thungapathra M, Gautam JK, Meena LS, Singh Y, Ghosh A, Tyagi AK, Sharma AK (2008a) Expression of accessory colonization factor subunit A (ACFA) of Vibrio cholerae and ACFA fused to cholera toxin B subunit in transgenic tomato (Solanum lycopersicum). J Biotechnol 135:22–27
Sharma MK, Singh NK, Jani D, Sisodia R, Thungapathra M, Gautam JK, Meena LS, Singh Y, Ghosh A, Tyagi AK, Sharma AK (2008b) Expression of toxin co-regulated pilus subunit A (TCPA) of Vibrio cholerae and its immunogenic epitopes fused to cholera toxin B subunit in transgenic tomato (Solanum lycopersicum). Plant Cell Rep 27:307–318
Shchelkunov SN, Salyaev RK, Pozdnyakov SG, Rekoslavskaya NI, Nesterov AE, Ryzhova TS, Sumtsova VM, Pakova NV, Mishutina UO, Kopytina TV, Hammond RW (2006) Immunogenicity of a novel, bivalent, plant-based oral vaccine against hepatitis B and human immunodeficiency viruses. Biotechnol Lett 28:959–967
Shin EA, Lee JY, Kim TG, Park YK, Langridge WH (2006) Synthesis and assembly of an adjuvanted Porphyromonas gingivalis fimbrial antigen fusion protein in plants. Protein Expr Purif 47:99–109
Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J (2002) Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med 196:1017–1024
Smith ML, Richter L, Arntzen CJ, Shuler ML, Mason HS (2003) Structural characterization of plant-derived hepatitis B surface antigen employed in oral immunization studies. Vaccine 21:4011–4021
Soria-Guerra RE, Rosales-Mendoza S, Márquez-Mercado C, López-Revilla L, Castillo-Collazo R, Alpuche-Solís AG (2007) Transgenic tomatoes express an antigenic polypeptide containing epitopes of the diphtheria, pertussis and tetanus exotoxins, encoded by a synthetic gene. Plant Cell Rep 26:961–968
Soria-Guerra RE, Alpuche-Solís AG, Rosales-Mendoza S, Moreno-Fierros L, Martínez-González L, Bendik E, Korban SS (2009) Expression of a multi-epitope DPT fusion protein in transplastomic tobacco plants retains both antigenicity and immunogenicity of all three components of the functional oligomer. Planta 229:1293–1302
Soria-Guerra RE, Rosales-Mendoza S, Moreno-Fierros L, Lopez-Revilla R, Alpuche-Solis AG (2011) Oral immunogenicity of tomato-derived sDPT polypeptide containing Corynebacterium diphtheriae, Bordetella pertussis and Clostridium tetani exotoxin epítopes. Plant Cell Rep 30:417–424
Spangler BD (1992) Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev 56:622–647
Stoger E, Ma JKC, Fischer R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotech 16:167–173
Titball RW, Leary SE (1998) Plague. British Medical Bulletin 54 pp. 625–633
Vázquez RI, Moreno-Fierros L, Neri-Bazán L, De La Riva GA, López-Revilla R (1999) Bacillus thuringiensis Cry1Ac protoxin is a potent systemic and mucosal adjuvant. Scand J Immunol 49:578–584
Vázquez-Padrón RI, Moreno-Fierros L, Neri-Bazán L, de la Riva GA, López-Revilla R (1999) Intragastric and intraperitoneal administration of Cry1Ac protoxin from Bacillus thuringiensis induces systemic and mucosal antibody responses in mice. Life Sci 64:1897–1912
Walmsley AM, Alvarez ML, Jin Y, Kirk DD, Lee SM, Pinkhasov J, Rigano MM, Arntzen CJ, Mason HS (2003) Expression of the B subunit of Escherichia coli heat-labile enterotoxin as a fusion protein in transgenic tomato. Plant Cell Rep 21:1020–1026
Welkos SL, Davis KM, Pitt LM, Worsham PL, Friedlander AM (1995) Studies on the contribution of the F1-capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib Microbiol Immunol 13:299–305
Williamson ED, Stephen ME, Griffin KF, Green M, Russel P, Leary SEC, Oyston PCF, Easterbrook T, Reddin KM, Robinson A, Titball RW (1995) A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol Med Microbiol 12:223–230
Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW (1997) A sub-unit vaccine elicits IgG in serum, spleen cell culture and bronchial washing and protects immunized animals against pneumonic plague. Vaccine 15:1079–1084
Yu J, Langridge WH (2001) A plant-based multicomponent vaccine protects mice from enteric diseases. Nat Biotechnol 19:548–552
Zhang B, Yang YH, Lin YM, Rao Q, Zheng GG, Wu KF (2003) Expression and production of bioactive human interleukin-18 in transgenic tobacco plants. Biotechnol Lett 25:1629–1635
Zhou F, Badillo-Corona JA, Karcher D, Gonzalez-Rabade N, Piepenburg K, Borchers AM, Maloney A, Kavanagh TA, Gray JC, Bock R (2008) High-level expression of human immunodeficiency virus antigens from the tobacco and tomato plastid genomes. Plant Biotech J 6:897–913
Zimmermann J, Saalbach I, Jahn D, Giersberg M, Haehnel S, Wedel J, Macek J, Zoufal K, Glünder G, Falkenburg D, Kipriyanov SM (2009) Antibody expressing pea seeds as fodder for prevention of gastrointestinal parasitic infections in chickens. BMC Biotechnology 9:74
Acknowledgments
Current investigations from the group are supported in part by CONACYT/México (grant CB-2008-01, 102109) and PROMEP/SEP/México (grant 103.5/10/5460).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Reski.
Rights and permissions
About this article
Cite this article
Soria-Guerra, R.E., Moreno-Fierros, L. & Rosales-Mendoza, S. Two decades of plant-based candidate vaccines: a review of the chimeric protein approaches. Plant Cell Rep 30, 1367–1382 (2011). https://doi.org/10.1007/s00299-011-1065-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1065-3