Abstract
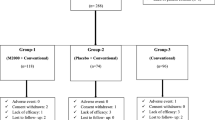
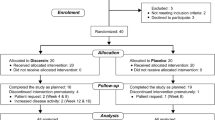
The aim of our study was to evaluate the clinical efficacy, safety, and tolerability of ornidazole in patients with rheumatoid arthritis (RA). This was 3 months, randomized, double-blind,placebo-controlled study. A total of 160 patients with active RA were randomly assigned to receive 1,000 mg ornidazole (n = 53), 500 mg ornidazole (n = 55), or placebo (n = 52). A significantly greater percentage of patients treated with 1,000 mg ornidazole met the American College of Rheumatology 20% improvement criteria (achieved an ACR20 response) at 3 months compared with patients who received placebo (62.0 vs. 32.4%; P < 0.001). Greater percentages of patients treated with 1,000 mg ornidazole also achieved ACR50 responses (38.3 vs. 10.9%; P < 0.001) and ACR70 responses (19.6 vs. 1.2%; P < 0.001) compared with patients who received placebo. Ornidazole treatment was also associated with significant reductions in pain and duration of morning stiffness, significant improvement in the quality of life and both the physician’s and patient’s global assessments, and significant reductions in disease activity as assessed by objective laboratory measures (erythrocyte sedimentation rate and C-reactive protein level). Ornidazole was well tolerated. There were no dose-limiting toxic effects. In this 3-month-trial ornidazole was safe, well tolerated, and associated with improvement in the inflammatory symptoms of RA.
Similar content being viewed by others
References
Albani S, Carson DA (1997) Etiology and pathogenesis of rheumatoid arthritis. In Koopman WJ (ed) Arthritis and allied conditions. Williams and Wilkins, Baltimore, pp 979–992
Mercado FB, Marshall RI, Klestov AC, Bartold PM (2001) Relationship between rheumatoid arthritis and periodontitis. J Periodontol 72:779–787
Greenwald RA, Kirkwood K (1999) Adult periodontitis as a model for rheumatoid arthritis (with emphasis on treatment strategies). J Rheumatol 26:1650–1653
Ogrendik M, Kokino S, Ozdemir F, Bird PS, Hamlet S. Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. URL: http://www.medscape.com/viewarticle/505458. Accessed 2005 June 16
Moen K, Brun JG, Madland TM, Tynning T, Jonsson R (2003) Immunoglobulin G and A antibody responses to Bacteroides forsythus and Prevotella intermedia in sera and synovial fluids of arthritis patients. Clin Diagn Lab Immunol 10:1043–1050
Hannonen P, Mottonen T, Hakola M, Oka M (1993) Sulfasalazine in early rheumatoid arthritis. A 48-week double-blind, prospective, placebo-controlled study. Arthritis Rheum 36:1501–1509
Pinals RS, Kaplan SB, Lawson JG, Hepburn B (1986) Sulfasalazine in rheumatoid arthritis. A double-blind, placebo-controlled trial. Arthritis Rheum 29:1427–1434
O’Dell JR, Blakely KW, Mallek JA, Eckhoff PJ, Leff RD, Wees SJ et al (2001) Treatment of early seropositive rheumatoid arthritis: a two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis Rheum 44:2235–2241
Kanerud L, Scheynius A, Nord CE, Hafstrom I (1994) Effect of sulphasalazine on gastrointestinal microflora and on mucosal heat shock protein expression in patients with rheumatoid arthritis. Br J Rheumatol 33:1039–1048
Bartlett JG (1982) Anti-anaerobic antibacterial agents. Lancet 2:478–481
Jokipii AM, Jokipii L (1981) Metronidazole, tinidazole, ornidazole and anaerobic infections of the middle ear, maxillary sinus and central nervous system. Scand J Infect Dis Suppl 26:123–129
Kargul B, Kadir T (2001) The antibacterial effects of ornidazole on primary molars with infected pulps. Chemotherapy 47:203–207
Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL et al (1997) Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (pg75)-Fc fusion protein. N Engl J Med 337:141–147
Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI et al (1999) A trial of etanercept, a recombinant tumour necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 340:253–259
Seymour GJ, Gemmell E (2001) Cytokines in periodontal disease: where to from here? Acta Odontol Scand 59:167–173
Yoshimura A, Hara Y, Kaneko T, Kato I (1997) Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J Periodontal Res 32:279–286
Kjeldsen M, Holmstrup P, Lindemann RA, Bendzen K (1995) Bacterial-stimulated cytokine production of peripheral mononuclear cells from patients of various periodontitis categories. J Periodontol 66:139–144
Rossano F, Rizzo A, Sanges MR, Cipollaro de L’Ero G, Tufano MA (1993) Human monocytes and gingival fibroblasts release tumor necrosis factor-alpha, interleukin-1 alpha and interleukin-6 in response to particulate and soluble fractions of Prevotella melaninogenica and Fusobacterium nucleatum. Int J Clin Lab Res 23:165–168
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP (1983) Assessment of patient satisfaction in activities of daily using a modified Stanford health assessment questionnaire. Arthritis Rheum 26:1346–1353
Fries JF, Spitz PW, Young DY (1982) The dimensions of health outcomes: the health assessment questionnaire, disability and pain scale. J Rheumatol 9:789–793
Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B et al (1993) The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum 36:729–740
Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C et al (1995) American collage of rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 38:727–735
Felson DT, Anderson JJ, Lange ML, Wells G, LaValley MP (1998) Should improvement in rheumatoid arthritis clinical trials be defined as fifty percent or seventy percent improvement in core set measures, rather than twenty percent? Arthritis Rheum 41:1564–1570
Jousimies-Somer HR, Finegold SM (1991). Anaerobic gram negative bacilli and cocci. In: Balows A, Hausler WJ, Herrmann KL, Isenberg HD, Shadomy HJ (eds) Manual of clinical microbiology. American Society for Microbiology, Washington DC, pp 538–553
Lorber B (2000) Bacteroides, Prevotella, Porphyromonas and Fusobacterium species (and other medically important anaerobic gram-negative bacilli). In Mandell GL, Bennett JE, Dolin R (eds) Mandell, Douglas and Bennett’s principles and practice of infectious Diseases. Churchill Livingstone, Philadelphia, pp 2561–2570
Marsh P, Martin MV (eds) (2001) Oral microbiology. MPG Books, Bodmin
Zhou Z, Menard HA (2002) Autoantigenic posttranslational modifications of proteins: does it apply to rheumatoid arthritis? Curr Opin Rheumatol 14:250–253
Tighe H, Carson DA (2001) Rheumatoid factor. In: Ruddy S, Harris ED Jr, Sledge CB (eds) Kelley’s textbook of rheumatology. W.B Saunders, Philadelphia, pp 151–160
Bonagura VR, Artandi SE, Davidson A, Randen I, Agostino N, Thompson K et al (1993) Mapping studies reveal unique epitopes on IgG recognized by rheumatoid arthritis- derived monoclonal rheumatoid factors. J Immunol. 151:3840–3852
Martin T, Crouzier R, Weber JC, Kipps TJ, Pasquali JL (1994) Structure-function studies on a polyreactive (natural) autoantibody. Polyreactivity is dependent on somatically generated sequences in the third complementarity-determining region of the antibody heavy chain. J Immunol. 152:5988–5996
The J, Ebersole JL (1991). Rheumatoid factor (RF) distribution in periodontal disease. J Clin Immunol 11:132–142
The J, Ebersole JL (1996). Rheumatoid factor from periodontitis patients cross-reacts with epitopes on oral bacteria. Oral Dis 2:253–262
Yoshida A, Nakano Y, Yamashita Y, Oho T, Ito H, Kondo M et al (2001) Immunodominant region of Actinobacillus actinomycetemcomitans 40-kilodalton heat shock protein in patients with rheumatoid arthritis. J Dent Res 80:346–350
Maeda H, Miyamoto M, Kokeguchi S, Kono T, Nishimura F, Takashiba S et al (2000) Epitope mapping of heat shock protein 60 (GroEL) from Porphyromonas gingivalis. FEMS Immunol Med Microbiol 28:219–224
Ando T, Kato T, Ishihara K, Ogiuchi H, Okuda K (1995) Heat shock proteins in the human periodontal disease process. Microbiol Immunol. 39:321–327
Ueki K, Tabeta K, Yoshie H, Yamazaki K (2002) Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol 127:72–77
Schett G, Redlich K, Xu Q, Bizan P, Groger M, Tohidast-Akrad M et al (1998) Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest 102:302–311
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogrendik, M. Treatment of rheumatoid arthritis with ornidazole: a randomized, double-blind, placebo-controlled study. Rheumatol Int 26, 1132–1137 (2006). https://doi.org/10.1007/s00296-006-0145-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-006-0145-0