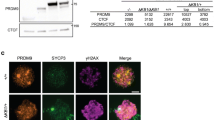
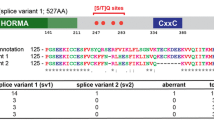
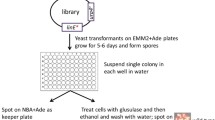
Abstract
DNA double-strand breaks (DSBs) initiate meiotic recombination in Schizosaccharomyces pombe and in other organisms. The Rec12 protein catalyzes the formation of these DSBs in concert with a multitude of accessory proteins the role of which in this process remains to be discovered. In an all-to-all yeast two-hybrid matrix analysis, we discovered new interactions among putative members of the meiotic recombination initiation complex. We found that Rec7, an axial-element associated protein with homologies to Saccharomyces cerevisiae Rec114, is interacting with Rec24. Rec7 and Rec24 also co-immunoprecipitate in S. pombe during meiosis. An amino acid change in a conserved, C-terminal phenylalanine in Rec7, F325A interrupts the interaction with Rec24. Moreover, rec7F325A shows a recombination deficiency comparable to rec7Δ. Another interaction was detected between Rec12 and Rec14, the orthologs of which in S. cerevisiae Spo11 and Ski8 interact accordingly. Amino acid changes Rec12Q308A and Rec12R309A disrupt the interaction with Rec14, like the according amino acid changes Spo11Q376A and Spo11RE377AA loose the interaction with Ski8. Both amino acid changes in Rec12 reveal a recombination deficient rec12 − phenotype. We propose that both Rec7–Rec24 and Rec12–Rec14 form subcomplexes of the meiotic recombination initiation complex.







Similar content being viewed by others
References
Arora C, Kee K, Maleki S, Keeney S (2004) Antiviral protein Ski8 is a direct partner of Spo11 in meiotic DNA break formation, independent of its cytoplasmic role in RNA metabolism. Mol Cell 13(4):549–559
Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K (2009) Current Protocols in Molecular Biology. 5 vols, vol 4. John Wiley & Sons, Inc
Bähler J, Wyler T, Loidl J, Kohli J (1993) Unusual nuclear structures in meiotic prophase of fission yeast: a cytological analysis. J Cell Biol 121(2):241–256
Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14(10):943–951
Basi G, Schmid E, Maundrell K (1993) Tata box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123(1):131–136
Beach D (1985) Sexual differentiation is controlled by a protein kinase encoded by the ran1+ gene in fission yeast. Cold Spring Harb Symp Quant Biol 50:635–641
Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P (1997) An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature 386(6623):414–417
Bishop DK, Nikolski Y, Oshiro J, Chon J, Shinohara M, Chen X (1999) High copy number suppression of the meiotic arrest caused by a dmc1 mutation: Rec114 imposes an early recombination block and rad54 promotes a dmc1-independent DSB repair pathway. Genes Cells 4(8):425–444
Borde V (2007) The multiple roles of the Mre11 complex for meiotic recombination. Chromosome Res 15(5):551–563
Carballo JA, Johnson AL, Sedgwick SG, Cha RS (2008) Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell 132(5):758–770
Caspari T, Dahlen M, Kanter-Smoler G, Lindsay HD, Hofmann K, Papadimitriou K, Sunnerhagen P, Carr AM (2000) Characterization of Schizosaccharomyces pombe Hus1: a pcna-related protein that associates with Rad1 and Rad9. Mol Cell Biol 20(4):1254–1262
Cervantes MD, Farah JA, Smith GR (2000) Meiotic DNA breaks associated with recombination in s. pombe. Mol Cell 5(5):883–888
Cheng Z, Liu Y, Wang C, Parker R, Song H (2004) Crystal structure of Ski8p, a WD-repeat protein with dual roles in mRNA metabolism and meiotic recombination. Protein Sci 13(10):2673–2684
Chikashige Y, Kurokawa R, Haraguchi T, Hiraoka Y (2004) Meiosis induced by inactivation of Pat1 kinase proceeds with aberrant nuclear positioning of centromeres in the fission yeast Schizosaccharomyces pombe. Genes Cells 9(8):671–684
Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y (2006) Meiotic proteins Bqt1 and Bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125(1):59–69
Davis L, Smith GR (2003) Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics 163(3):857–874
Davis L, Rozalen AE, Moreno S, Smith GR, Martin-Castellanos C (2008) Rec25 and Rec27, novel linear-element components, link cohesin to meiotic DNA breakage and recombination. Curr Biol 18(11):849–854
De Veaux LC, Hoagland NA, Smith GR (1992) Seventeen complementation groups of mutations decreasing meiotic recombination in Schizosaccharomyces pombe. Genetics 130(2):251–262
Deutschbauer AM, Jaramillo DF, Proctor M, Kumm J, Hillenmeyer ME, Davis RW, Nislow C, Giaever G (2005) Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 169(4):1915–1925
Doll E, Molnar M, Hiraoka Y, Kohli J (2005) Characterization of Rec15, an early meiotic recombination gene in Schizosaccharomyces pombe. Curr Genet 48(5):323–333
Evans DH, Li YF, Fox ME, Smith GR (1997) A WD repeat protein, Rec14, essential for meiotic recombination in Schizosaccharomyces pombe. Genetics 146(4):1253–1264
Fox ME, Smith GR (1998) Control of meiotic recombination in Schizosaccharomyces pombe. Prog Nucleic Acid Res Mol Biol 61:345–378
Gregan J, Rabitsch PK, Sakem B, Csutak O, Latypov V, Lehmann E, Kohli J, Nasmyth K (2005) Novel genes required for meiotic chromosome segregation are identified by a high-throughput knockout screen in fission yeast. Curr Biol 15(18):1663–1669
Gutz H, Heslot H, Leupold U, Loprieno N (1974) Schizosaccharomyces pombe. Handb Genet 1:395–446
Henderson KA, Kee K, Maleki S, Santini PA, Keeney S (2006) Cyclin-dependent kinase directly regulates initiation of meiotic recombination. Cell 125(7):1321–1332
Hudson JRJ, Dawson EP, Rushing KL, Jackson CH, Lockshon D, Conover D, Lanciault C, Harris JR, Simmons SJ, Rothstein R, Fields S (1997) The complete set of predicted genes from Saccharomyces cerevisiae in a readily usable form. Genome Res 7(12):1169–1173
James P, Halladay J, Craig EA (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144(4):1425–1436
Jiao K, Salem L, Malone R (2003) Support for a meiotic recombination initiation complex: interactions among Rec102p, Rec104p, and Spo11p. Mol Cell Biol 23(16):5928–5938
Kee K, Keeney S (2002) Functional interactions between Spo11 and Rec102 during initiation of meiotic recombination in Saccharomyces cerevisiae. Genetics 160(1):111–122
Kee K, Protacio RU, Arora C, Keeney S (2004) Spatial organization and dynamics of the association of Rec102 and Rec104 with meiotic chromosomes. Embo J 23(8):1815–1824
Keeney S (2001) Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol 52:1–53
Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88(3):375–384
Kitajima TS, Yokobayashi S, Yamamoto M, Watanabe Y (2003) Distinct cohesin complexes organize meiotic chromosome domains. Science 300(5622):1152–1155
Latypov V, Rothenberg M, Lorenz A, Octobre G, Csutak O, Lehmann E, Loidl J, Kohli J (2010) The roles of Hop1 and Mek1 in meiotic chromosome pairing and recombination-partner choice in Schizosaccharomyces pombe. Mol Cell Biol. doi:10.1128/MCB.00919-09
Li J, Hooker GW, Roeder GS (2006) Saccharomyces cerevisiae Mer2, Mei4 and Rec114 form a complex required for meiotic double-strand break formation. Genetics 173(4):1969–1981
Lichten M (2008) Meiotic chromatin: the substrate for recombination initiation. In: Egel R, Lankenau D-H (eds) Recombination and meiosis—models, means, and evolution vol 3. Springer, Berlin, Heidelberg, pp 165–193
Lorenz A, Wells JL, Pryce DW, Novatchkova M, Eisenhaber F, McFarlane RJ, Loidl J (2004) S pombe meiotic linear elements contain proteins related to synaptonemal complex components. J Cell Sci 117(Pt 15):3343–3351
Lorenz A, Estreicher A, Kohli J, Loidl J (2006) Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe. Chromosoma 115(4):330–340. doi:10.1007/s00412-006-0053-9
Ludin K, Jiang R, Carlson M (1998) Glucose-regulated interaction of a regulatory subunit of protein phosphatase 1 with the Snf1 protein kinase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 95(11):6245–6250
Ludin K, Mata J, Watt S, Lehmann E, Bahler J, Kohli J (2008) Sites of strong Rec12/Spo11 binding in the fission yeast genome are associated with meiotic recombination and with centromeres. Chromosoma 117(5):431–444
Madrona AY, Wilson DK (2004) The structure of Ski8p, a protein regulating mRNA degradation: Implications for WD protein structure. Protein Sci 13(6):1557–1565
Maleki S, Neale MJ, Arora C, Henderson KA, Keeney S (2007) Interactions between Mei4, Rec114, and other proteins required for meiotic DNA double-strand break formation in Saccharomyces cerevisiae. Chromosoma 116(5):471–486
Mao-Draayer Y, Galbraith AM, Pittman DL, Cool M, Malone RE (1996) Analysis of meiotic recombination pathways in the yeast Saccharomyces cerevisiae. Genetics 144(1):71–86
Martin-Castellanos C, Blanco M, Rozalen AE, Perez-Hidalgo L, Garcia AI, Conde F, Mata J, Ellermeier C, Davis L, San-Segundo P, Smith GR, Moreno S (2005) A large-scale screen in S pombe identifies seven novel genes required for critical meiotic events. Curr Biol 15(22):2056–2062
Matsumoto Y, Sarkar G, Sommer SS, Wickner RB (1993) A yeast antiviral protein, Ski8, shares a repeated amino acid sequence pattern with beta-subunits of g proteins and several other proteins. Yeast 9(1):43–51
Molnar M, Bähler J, Sipiczki M, Kohli J (1995) The rec8 gene of Schizosaccharomyces pombe is involved in linear element formation, chromosome pairing and sister-chromatid cohesion during meiosis. Genetics 141(1):61–73
Molnar M, Bähler J, Kohli J, Hiraoka Y (2001a) Live observation of fission yeast meiosis in recombination-deficient mutants: a study on achiasmate chromosome segregation. J Cell Sci 114(Pt 15):2843–2853
Molnar M, Parisi S, Kakihara Y, Nojima H, Yamamoto A, Hiraoka Y, Bozsik A, Sipiczki M, Kohli J (2001b) Characterization of Rec7, an early meiotic recombination gene in Schizosaccharomyces pombe. Genetics 157(2):519–532
Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194:795–823
Neale MJ, Pan J, Keeney S (2005) Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature 436(7053):1053–1057
Nurse P, Thuriaux P, Nasmyth K (1976) Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 146(2):167–178
Parisi S, McKay MJ, Molnar M, Thompson MA, van der Spek PJ, van Drunen-Schoenmaker E, Kanaar R, Lehmann E, Hoeijmakers JH, Kohli J (1999) Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol Cell Biol 19(5):3515–3528
Ponticelli AS, Smith GR (1989) Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics 123(1):45–54
Prieler S, Penkner A, Borde V, Klein F (2005) The control of Spo11’s interaction with meiotic recombination hotspots. Genes Dev 19(2):255–269
Puizina J, Siroky J, Mokros P, Schweizer D, Riha K (2004) Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell 16(8):1968–1978
Ridley SP, Sommer SS, Wickner RB (1984) Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of m2 double-stranded RNA by l-a-hn and confer cold sensitivity in the presence of m and l-a-hn. Mol Cell Biol 4(4):761–770
Rothenberg M, Kohli J, Ludin K (2009) Ctp1 and the MRN-complex are required for endonucleolytic Rec12 removal with release of a single class of oligonucleotides in fission yeast. PLoS Genet 5(11):e1000,722
Salem L, Walter N, Malone R (1999) Suppressor analysis of the Saccharomyces cerevisiae gene rec104 reveals a genetic interaction with rec102. Genetics 151(4):1261–1272
Sasanuma H, Murakami H, Fukuda T, Shibata T, Nicolas A, Ohta K (2007) Meiotic association between Spo11 regulated by Rec102, Rec104 and Rec114. Nucleic Acids Res 35(4):1119–1133
Sasanuma H, Hirota K, Fukuda T, Kakusho N, Kugou K, Kawasaki Y, Shibata T, Masai H, Ohta K (2008) Cdc7-dependent phosphorylation of Mer2 facilitates initiation of yeast meiotic recombination. Genes Dev 22(3):398–410
Smith TF, Gaitatzes C, Saxena K, Neer EJ (1999) The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 24(5):181–185
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg JM (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403(6770):623–627
Wan L, Niu H, Futcher B, Zhang C, Shokat KM, Boulton SJ, Hollingsworth NM (2008) Cdc28-Clb5 (CDK-S) and Cdc7-Dbf4 (DDK) collaborate to initiate meiotic recombination in yeast. Genes Dev 22(3):386–397
Xu L, Weiner BM, Kleckner N (1997) Meiotic cells monitor the status of the interhomolog recombination complex. Genes Dev 11(1):106–118
Yamamoto A, Hiraoka Y (2003) Monopolar spindle attachment of sister chromatids is ensured by two distinct mechanisms at the first meiotic division in fission yeast. Embo J 22(9):2284–2296
Young JA, Schreckhise RW, Steiner WW, Smith GR (2002) Meiotic recombination remote from prominent DNA break sites in S pombe. Mol Cell 9(2):253–263
Young JA, Hyppa RW, Smith GR (2004) Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics 167(2):593–605
Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33:603–754
Acknowledgments
We thank Marc-David Ruepp and Kathleen Gould for plasmids, Valerie Borde, Cristina Martin-Castellanos, Jurai Gregan, and Franz Klein for helpful discussions. Our acknowledgements for S. cerevisiae strains and Y2H plasmids go to T. N. Davis, grant P41 RR11823 from the National Center for Research Resources at the National Institutes of Health. The study was supported by the Swiss National Science Foundation to JK and KL. KL was supported by the UniBern Forschungstiftung and SS by a short-term fellowship of the Boehringer Ingelheim Fonds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Yamamoto.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Steiner, S., Kohli, J. & Ludin, K. Functional interactions among members of the meiotic initiation complex in fission yeast. Curr Genet 56, 237–249 (2010). https://doi.org/10.1007/s00294-010-0296-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-010-0296-0