Abstract
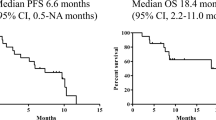
Purpose: We administered chemotherapy consisting of a combination of 5-day continuous infusion of cisplatin (20 mg/m2 per day) plus irinotecan (160 mg/m2 per day, as a bolus, on day 1) with recombinant human granulocyte colony-stimulating factor (rG-CSF) support to previously untreated advanced non-small-cell lung cancer (NSCLC) patients, and evaluated the effectiveness and safety of this therapy. Patients: Enrolled in the study were 41 NSCLC patients. Results: Of the 41 patients, 24 achieved a partial response. The response rate was 58.5% (95% confidence interval, 42.2% to 74.8%), with a median response duration of 32.1 weeks. The median survival time was 44.8 weeks and the 1-year survival rate was 44%. A total of 100 courses of therapy were given. The major toxic effects were grade 3 or 4 diarrhea (23%), granulocytopenia (20%), thrombocytopenia (15%) and anemia (15%). There were no treatment-related deaths. Conclusions: Combination chemotherapy with irinotecan plus infusional cisplatin with rG-CSF support was well tolerated and effective in patients with advanced NSCLC.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 4 May 1998 / Accepted: 18 September 1998
Rights and permissions
About this article
Cite this article
Mori, K., Machida, S., Yoshida, T. et al. A phase II study of irinotecan and infusional cisplatin with recombinant human granulocyte colony-stimulating factor support for advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 43, 467–470 (1999). https://doi.org/10.1007/s002800050925
Issue Date:
DOI: https://doi.org/10.1007/s002800050925