Abstract
Purpose
poly(ADP ribose) polymerase inhibition has been shown to potentiate the cytotoxicity of DNA damaging agents. A phase I study of rucaparib and temozolomide showed that full-dose temozolomide could be given during PARP inhibition. We report the results of a phase II study of intravenous rucaparib 12 mg/m2 and oral temozolomide 200 mg/m2 on days 1–5 every 28 days in patients with advanced metastatic melanoma.
Methods
Patients with chemotherapy naïve measurable metastatic melanoma, performance status ≤2 and good end-organ function were recruited. Treatment was given until progression. A two stage phase II design was used, with response rate the primary endpoint. Population pharmacokinetics and pharmacodynamics were also explored.
Results
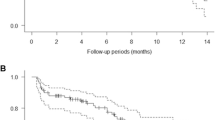
Forty-six patients were recruited with 37 patients receiving at least 2 cycles and 17 patients at least 6 cycles. Myelosuppression occurred with 25 patients (54 %) requiring a 25 % dose reduction in temozolomide. The response rate was 17.4 %, median time to progression 3.5 months, median overall survival 9.9 months, and 36 % of patients were progression-free at 6 months.
Conclusions
This study showed that temozolomide (150–200 mg/m2/day) can safely be given with a PARP inhibitory dose of rucaparib, increasing progression-free survival over historical controls in metastatic melanoma patients.



Similar content being viewed by others
References
Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG (2005) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. 17th EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics, Philadelphia, PA, pp 2139–2146
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251
Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG (2006) Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol 24:4738–4745
Bernstein C, Bernstein H, Payne CM, Garewal H (2002) DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res 511:145–178
Cancer Research UK (2006) www.cancerresearchuk.org
de Murcia G, Menissier de Murcia J (1994) Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci 19:172–176
Eigentler TK, Caroli UM, Radny P, Garbe C (2003) Palliative therapy of disseminated malignant melanoma: a systemic review of 41 randomised clinical trials. Lancet Oncol 4:748–759
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819
Gajewski TF, Sosman J, Gerson SL, Liu L, Dolan E, Lin S, Vokes EE (2005) Phase II trial of the O6-alkylguanine DNA alkyltransferase inhibitor O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea in advanced melanoma. Clin Cancer Res 11:7861–7865
Gelmon KA, Hirte HW, Robidoux A, Tonkin KS, Tischkowitz M, Swenerton K, Huntsman D, Carmichael J, MacPherson E, Oza AM (2010) Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer. J Clin Oncol 28(Suppl):Abstract 3002
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, Macpherson E, Carmichael J, Oza A (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12:852–861
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Huncharek M, Caubet JF, McGarry R (2001) Single-agent DTIC versus combination chemotherapy with or without immunotherapy in metastatic melanoma: a meta-analysis of 3273 patients from 20 randomized trials. Melanoma Res 11:75–81
Jewell R, Conway C, Mitra A, Randerson-Moor J, Lobo S, Nsengimana J, Harland M, Marples M, Edward S, Cook M, Powell B, Boon A, de Kort F, Parker KA, Cree IA, Barrett JH, Knowles MA, Bishop DT, Newton-Bishop J (2010) Patterns of expression of DNA repair genes and relapse from melanoma. Clin Cancer Res 16:5211–5221
Kirkwood JM, Lorigan P, Hersey P, Hauschild A, Robert C, McDermott D, Marshall MA, Gomez-Navarro J, Liang JQ, Bulanhagui CA Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 16:1042–1048
Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM (2008) Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26:527–534
Lorigan P, Eisen T, Hauschild A (2008) Systemic therapy for metastatic malignant melanoma–from deeply disappointing to bright future? Exp Dermatol 17:383–394
Margison G, Koref MS, Povey A (2002) Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis 17:483–487
Middleton M, Friedberg EC, Hamid O, Daud A, Plummer R, Schuster R, Qian J, Luo Y, Giranda VL, McArthur G (2011) Veliparib (ABT-888) plus temozolomide versus temozolomide alone: efficacy and safety in patients with metastatic melanoma in a randomized double-blind placebo-controlled trial. Pigment Cell Melanoma Res 24:1022–1023
Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N (2000) Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 18:158–166
Newlands ES, Stevens MFG, Wedge SR, Wheelhouse RT, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23:35–61
Patel PM, Suciu S, Mortier L, Kruit W, Robert C, Schadendorf D, Keilholz U, Musat E, Eggermont A, Spatz A (2008) Extended schedule, escalated dose temozlomide versus dacarbazine in stage IV malignant melanoma: final results of the randomised phase III study(Eortc 18032). 33rd European-Society-for-Medical-Oncology Congress, Stockholm, SWEDEN, p 3
Patel PM, Suciu S, Mortier L, Kruit WH, Robert C, Schadendorf D, Trefzer U, Punt CJ, Dummer R, Davidson N, Becker J, Conry R, Thompson JA, Hwu WJ, Engelen K, Agarwala SS, Keilholz U, Eggermont AM, Spatz A (2011) Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer 47:1476–1483
Plummer ER, Middleton MR, Jones C, Olsen A, Hickson I, McHugh P, Margison GP, McGown G, Thorncroft M, Watson AJ, Boddy AV, Calvert AH, Harris AL, Newell DR, Curtin NJ (2005) Temozolomide pharmacodynamics in patients with metastatic melanoma: DNA damage and activity of repair enzymes 6-alkylguanine alkyltransferase and poly(ADP-ribose) polymerase-1. Clin Cancer Res 11:3402–3409
Plummer R, Jones C, Middleton M, Wilson R, Evans J, Olsen A, Curtin N, Boddy A, McHugh P, Newell D, Harris A, Johnson P, Steinfeldt H, Dewji R, Wang D, Robson L, Calvert H (2008) Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res 14:7917–7923
Pocock S, Clayton T, Altman D (2002) Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet 359:1686–1689
Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE II, Bigner DD, Friedman HS (2005) Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol 23:7178–7187
Quinn JA, Pluda J, Dolan ME, Delaney S, Kaplan R, Rich JN, Friedman AH, Reardon DA, Sampson JH, Colvin OM, Haglund MM, Pegg AE, Moschel RC, McLendon RE, Provenzale JM, Gururangan S, Tourt-Uhlig S, Herndon JE II, Bigner DD, Friedman HS (2002) Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol 20:2277–2283
Ranson M, Hersey P, Thompson D, Beith J, McArthur G, Haydon A, Davis I, Kefford R, Mortimer P, Harris PA, Baka S, Seebaran A, Sabharwal A, Watson AJ, Margison G, Middleton M (2007) A randomised trial of the combination of lomeguatrib and temozolomide alone in patients with advanced melanoma. J Clin Oncol 25:2540–2545
Ranson M, Middleton MR, Bridgewater J, Lee SM, Dawson M, Jowle D, Halbert G, Waller S, McGrath H, Gumbrell L, McElhinney RS, Donnelly D, McMurry TB, Margison GP (2006) Lomeguatrib, a potent inhibitor of O6-alkylguanine-DNA-alkyltransferase: phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res 12:1577–1584
Schilsky RL, Dolan ME, Bertucci D, Ewesuedo RB, Vogelzang NJ, Mani S, Wilson LR, Ratain MJ (2000) Phase I clinical and pharmacological study of O6-benzylguanine followed by carmustine in patients with advanced cancer. Clin Cancer Res 6:3025–3031
Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG (2006) Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist 11:165–180
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376:235–244
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 17:2762–2771
Zaremba T, Cole M, Coulthard S, Plummer R, Curtin N (2008) Poly(ADP-ribose) polymerase-1 (PARP-1) genomics in relation to activity in healthy volunteers and cancer patients. AACR Meeting Abstracts:540
Zaremba T, Thomas HD, Cole M, Coulthard SA, Plummer ER, Curtin NJ (2011) Poly(ADP-ribose) polymerase-1 (PARP-1) pharmacogenetics, activity and expression analysis in cancer patients and healthy volunteers. Biochem J 436:671–679
Acknowledgments
We would like to thank all our patients and their families for participating in this research, and the research nurses and data managers at the clinical sites for their help and support. This clinical trial was sponsored and funded by Pfizer GRD. Staff working on this project at the 6 UK academic institutions are all supported by Cancer Research UK and the UK Departments of Health through the Experimental Cancer Medicine Centre initiative.
Conflict of interest
Calvert, Plummer, Lorigan, Steven, Middleton, Curtin, Wilson and Scott received research costs to support conduct of the study from Pfizer GRD. Wang, Dewji, Gallo and Abbattista are employees of Pfizer GRD. Calvert, Curtin and Plummer are named as inventors on patents pertaining to PF-01367338.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plummer, R., Lorigan, P., Steven, N. et al. A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother Pharmacol 71, 1191–1199 (2013). https://doi.org/10.1007/s00280-013-2113-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2113-1