Abstract
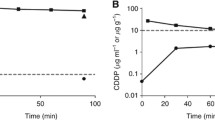
Ovarian cancer is the leading cause of gynecological cancer-related death in Western countries. The present treatment standards for ovarian cancer are based on the association of debulking surgery with platinum-based chemotherapy. Another strategy that could be further investigated is intraperitoneal chemotherapy (IP). We previously described that the 2-h administration of intraoperative IP cisplatin did not reach satisfactory concentrations. In the present study, we present the results of a pharmacokinetic analysis performed after two consecutive 1-h IP 30 mg/l cisplatin administrations. Twenty-seven patients with advanced epithelial cancer classified FIGO stage IIIC were included in the study. Blood and IP samples were taken over a 24-h period, during and after IP treatment. Both total and ultrafiltered (Uf) platinum (Pt) concentration levels were analyzed. Biological and clinical toxicities were also recorded. With this strategy, IP Pt concentrations stayed above the target concentration (10 mg/l) for a satisfactory length of time. The serum Pt concentrations were higher than those observed with the “one-bath” protocol and they induced the occurrence of recoverable renal toxicities (3 grade 1, 7 grade 2 and 4 grade 3). The best predictive parameter for renal failure was the total Pt 24-h Area Under the Curve (AUC) with a threshold value of 25 mg h/l RR = 0.31 (95% CI 0.13 − 0.49, P < 0.01). Administration of an increased amount of cisplatin is feasible and a satisfactory level of IP Pt concentrations is obtained. However, this improvement is associated with an increase in serum Pt levels and resulting renal toxicities. An attractive solution would be to decrease Pt transfer from peritoneum to bloodstream. A phase 1 study using intraoperative IP epinephrine in order to decrease this transfer is presently being carried out.




Similar content being viewed by others
References
Ahmad SA, Kim J, Sussman JJ, Soldano DA, Pennington LJ, James LE, Lowy AM (2004) Reduced morbidity following cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion. Ann Surg Oncol 11:387–392
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950–1955
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43
Bookman MA (2005) Standard treatment in advanced ovarian cancer in 2005: the state of the art. Int J Gynecol Cancer 15(Suppl 3):212–220
Chatelut E, de Forni M, Canal P, Chevreau C, Roche H, Plusquellec Y, Johnson NP, Houin G, Bugat R (1991) Teniposide and cisplatin given by intraperitoneal administration: preclinical and phase I/pharmacokinetic studies. Ann Oncol 2:217–221
Chauffert B, Favoulet P, Polycarpe E, Duvillard C, Beltramo JL, Bichat F, Rat P, Genne P, Benoit L (2003) Rationale supporting the use of vasoconstrictors for intraperitoneal chemotherapy with platinum derivatives. Surg Oncol Clin N Am 12:835–848
Chauffert B, Molucon-Chabrot C, Isambert N, Benoit L, Zanetta S, Fraisse J, Guilland JC, Royer B, Monin-Baroille P, Flesch M, Fargeot M, Coudert B, Mayer F, Fumoleau P (2006) Feasibility of using intraperitoneal epinephrine and cisplatin in patients with advanced peritoneal carcinomatosis. Anticancer Drugs 10:1211–1217
Cho HK, Lush RM, Bartlett DL, Alexander HR, Wu PC, Libutti SK, Lee KB, Venzon DJ, Bauer KS, Reed E, Figg WD (1999) Pharmacokinetics of cisplatin administered by continuous hyperthermic peritoneal perfusion (CHPP) to patients with peritoneal carcinomatosis. J Clin Pharmacol 39:394–401
du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, Bowtell D, Brady M, Casado A, Cervantes A, Eisenhauer E, Friedlaender M, Fujiwara K, Grenman S, Guastalla JP, Harper P, Hogberg T, Kaye S, Kitchener H, Kristensen G, Mannel R, Meier W, Miller B, Neijt JP, Oza A, Ozols R, Parmar M, Pecorelli S, Pfisterer J, Poveda A, Provencher D, Pujade-Lauraine E, Randall M, Rochon J, Rustin G, Sagae S, Stehman F, Stuart G, Trimble E, Vasey P, Vergote I, Verheijen R, Wagner U (2005) 2004 consensus statements on the management of ovarian cancer: final document of the 3rd international gynecologic cancer intergroup ovarian cancer consensus conference (GCIG OCCC 2004). Ann Oncol 16(Suppl 8):viii7–viii12
Goodman MT, Howe HL (2003) Descriptive epidemiology of ovarian cancer in the United States, 1992–1997. Cancer 97:2615–2630
Goodman MT, Howe HL, Tung KH, Hotes J, Miller BA, Coughlin SS, Chen VW (2003) Incidence of ovarian cancer by race and ethnicity in the United States, 1992–1997. Cancer 97:2676–2685
Jaaback K, Johnson N (2006) Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 1:CD005340
Kern W, Braess J, Kotschofsky M, Samel S, Becker H, Hiddemann W, Schleyer E (2002) Application of cisplatin as intraoperative hyperthermic peritoneal lavage (IHPL) in patients with locally advanced gastric cancer: analysis of pharmacokinetics and of nephrotoxicity. Anticancer Res 22:3099–3102
Markman M (2003) Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol 4:277–283
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the gynecologic oncology group, southwestern oncology group, and eastern cooperative oncology group. J Clin Oncol 19:1001–1007
Markman M, Walker JL (2006) Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol 24:988–994
Pignata S, Pisano C, Di Maio M, Iodice F, Casella G, Laurelli G, Greggi S, Iaffaioli RV (2006) Medical treatment of resistant or recurrent epithelial ovarian cancer. Ann Oncol 17(Suppl 7):vii49–vii50
Quinn M, Avall-Lundqvist E, du Bois A, Vermorken J, Parmar M, Pfisterer J, Stuart G, Thigpen T, Neijt J (2005) History, scope and methodology of the 3rd international consensus workshop on ovarian cancer 2004. Ann Oncol 16(Suppl 8):viii5–viii6
Royer B, Guardiola E, Polycarpe E, Hoizey G, Delroeux D, Combe M, Chaigneau L, Samain E, Chauffert B, Heyd B, Kantelip JP, Pivot X (2005) Serum and intraperitoneal pharmacokinetics of cisplatin within intraoperative intraperitoneal chemotherapy: influence of protein binding. Anticancer Drugs 16:1009–1016
Stuart GC (2003) First-line treatment regimens and the role of consolidation therapy in advanced ovarian cancer. Gynecol Oncol 90:S8–S15
van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, Beijnen JH, Bartelink H, Begg AC (1998) Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer 34:148–154
van Ruth S, Verwaal VJ, Zoetmulder FA (2003) Pharmacokinetics of intraperitoneal mitomycin C. Surg Oncol Clin N Am 12:771–780
Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA (2001) Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 27:365–374
Acknowledgments
The authors are very grateful to the staff of the pharmacology department for their help in samples management. This work was supported by a grant from the Ligue Contre le Cancer du Doubs. The authors of this manuscript also state that they have no conflict of interest in the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Royer, B., Delroeux, D., Guardiola, E. et al. Improvement in intraperitoneal intraoperative cisplatin exposure based on pharmacokinetic analysis in patients with ovarian cancer. Cancer Chemother Pharmacol 61, 415–421 (2008). https://doi.org/10.1007/s00280-007-0484-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0484-x