Abstract
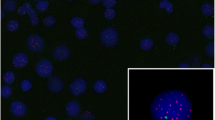
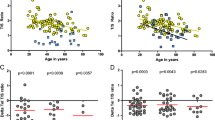
Telomere shortening with age may lead to genomic instability and an increased risk of cancer. Given the role of the microenvironment in the pathophysiology of the myelodysplastic syndrome (MDS), primarily a disease of older age, we determined telomere length in primary cultured marrow stroma cells using quantitative fluorescent in situ hybridization (qFISH) and quantitative polymerase chain reaction (qPCR). qFISH showed comparable rates of decrease in telomere length with age in MDS patients and age-matched healthy controls. Telomere length assessment by qPCR showed similar results. These findings suggest a lack of significant differences between MDS patients and healthy controls in terms of telomere stability in marrow stroma in contrast to that observed in hematopoietic cells. In conclusion, this demonstrates that, although MDS stroma cells and hematopoietic cells share the same microenvironment, the stromal cells do not share the processes that contribute to accelerated telomere attrition, suggesting that stromal cell proliferative potential is not limiting in MDS.


Similar content being viewed by others
References
Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406:641–645 doi:10.1038/35020592
Awaya N, Rupert K, Bryant E, Torok-Storb B (2002) Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol 30:937–942 doi:10.1016/S0301-472X(02)00821-4
Baird DM, Kipling D (2004) The extent and significance of telomere loss with age. Ann N Y Acad Sci 1019:265–268 (review) doi:10.1196/annals.1297.044
Bitan M, Or R, Shapira MY, Resnick IB, Gesundheit B, Ackerstein A, Samuel S, Elad S, Slavin S (2008) Time to engraftment following allogeneic stem cell transplantation is significantly longer in patients with myelodysplastic syndrome than with acute myeloid leukemia. Bone Marrow Transplant 41:69–78 doi:10.1038/sj.bmt.1705878
Boultwood J, Fidler C, Kusec R, Rack K, Elliott PJ, Atoyebi O, Chapman R, Oscier DG, Wainscoat JS (1997) Telomere length in myelodysplastic syndromes. Am J Hematol 56:266–271 doi:10.1002/(SICI)1096-8652(199712)56:4<266::AID-AJH12>3.0.CO;2-7
Fern L, Pallis M, Ian CG, Seedhouse C, Russell N, Byrne J (2004) Clonal haemopoiesis may occur after conventional chemotherapy and is associated with accelerated telomere shortening and defects in the NQO1 pathway; possible mechanisms leading to an increased risk of t-AML/MDS. Br J Haematol 126:63–71 doi:10.1111/j.1365-2141.2004.05006.x
Flores-Figueroa E, Arana-Trejo RM, Gutierrez-Espindola G, Perez-Cabrera A, Mayani H (2005) Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res 29:215–224 doi:10.1016/j.leukres.2004.06.011
Goerner M, Roecklein B, Torok-Storb B, Heimfeld S, Kiem H-P (2000) Expansion and transduction of nonenriched human cord blood cells using HS-5 conditioned medium and FLT3-L. J Hematother Stem Cell Res 9:759–765 doi:10.1089/15258160050196803
Graf L, Iwata M, Torok-Storb B (2002) Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a. Blood 100:1509–1511 (Letter to Editor) doi:10.1182/blood-2002-03-0844
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088 (erratum appears in Blood 1998 Feb 1;91(3):1100)
Kerbauy DMB, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ (2004) Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-β2microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood 104:2202–2203 (Letter to the Editor) doi:10.1182/blood-2004-04-1518
Lesnikov V, Lesnikova M, Deeg HJ (2001) Pro-apoptotic and anti-apoptotic effects of transferrin and transferrin-derived glycans on hematopoietic cells and lymphocytes. Exp Hematol 29:477–489 doi:10.1016/S0301-472X(00)00687-1
Marcondes AM, Mhyre AJ, Stirewalt DL, Kim S-H, Dinarello CA, Deeg HJ (2008) Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci USA 105:2865–2870 doi:10.1073/pnas.0712391105
Maser RS, DePinho RA (2002) Connecting chromosomes, crisis, and cancer. Science 297:565–569 (review) doi:10.1126/science.297.5581.565
O’Sullivan JN, Finley JC, Risques RA, Shen WT, Gollahon KA, Moskovitz AH, Gryaznov S, Harley CB, Rabinovitch PS (2004) Telomere length assessment in tissue sections by quantitative FISH: image analysis algorithms. Cytometry A 58:120–131
Ohyashiki JH, Iwama H, Yahata N, Ando K, Hayashi S, Shay JW, Ohyashiki K (1999) Telomere stability is frequently impaired in high-risk groups of patients with myelodysplastic syndromes. Clin Cancer Res 5:1155–1160
Ohyashiki JH, Ohyashiki K, Fujimura T, Kawakubo K, Shimamoto T, Iwabuchi A, Toyama K (1994) Telomere shortening associated with disease evolution patterns in myelodysplastic syndromes. Cancer Res 54:3557–3560
Ohyashiki K, Iwama H, Yahata N, Tauchi T, Kawakubo K, Shimamoto T, Ohyashiki JH (2001) Telomere dynamics in myelodysplastic syndromes and acute leukemic transformation. Leuk Lymphoma 42:291–299 (review) doi:10.3109/10428190109064585
Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, Ayub K, Gallaher JL, Reid BJ, Rabinovitch PS (2007) Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomark Prev 16:2649–2655 doi:10.1158/1055-9965.EPI-07-0624
Sashida G, Ohyashiki JH, Nakajima A, Sumi M, Kawakubo K, Tauchi T, Ohyashiki K (2003) Telomere dynamics in myelodysplastic syndrome determined by telomere measurement of marrow metaphases. Clin Cancer Res 9:1489–1496
Scott BL, Deeg HJ Hematopoietic cell transplantation for acquired nonmalignant diseases and myelodysplastic syndrome. In: Hoffman R (ed) Hematology: basic principles and practice. Churchill Livingstone, New York
Sieglova Z, Zilovcova S, Cermak J, Rihova H, Brezinova D, Dvorakova R, Markova M, Maaloufova J, Sajdova J, Brezinova J, Zemanova Z, Michalova K (2004) Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis? Leuk Res 28:1013–1021 doi:10.1016/j.leukres.2003.11.020
Soenen-Cornu V, Tourino C, Bonnet ML, Guillier M, Flamant S, Kotb R, Bernheim A, Bourhis JH, Preudhomme C, Fenaux P, Turhan AG (2005) Mesenchymal cells generated from patients with myelodysplastic syndromes are devoid of chromosomal clonal markers and support short- and long-term hematopoiesis in vitro. Oncogene 24:2441–2448 doi:10.1038/sj.onc.1208405
Stirewalt DL, Mhyre AJ, Marcondes M, Pogosova-Agadjanyan E, Abbasi N, Radich JP, Deeg HJ (2008) Tumour necrosis factor-induced gene expression in human marrow stroma: clues to the pathophysiology of MDS? Br J Haematol 140:444–453 doi:10.1111/j.1365-2141.2007.06923.x
Acknowledgments
The authors thank Emily Spaulding, for technical assistance and Helen Crawford and Bonnie Larson for help with manuscript preparation.
Authors’ contributions
Mario Marcondes designed the experiments, conducted the experiments, and wrote the manuscript. Steve Bair contributed to the analysis. Peter Rabinovitch provided reagents, made suggestions to the experimental design, assisted in interpreting the results, and revised the manuscript. Ted Gooley carried out the statistical analysis. H. Joachim Deeg provided marrow samples, assisted in analyzing the results, and provided revisions to the manuscript. Rosana Risques contributed technical expertise. This study was supported in part by grant nos. HL082941, HL36444, AG13280, and K23 from the National Institutes of Health (NIH) Bethesda, MD, USA. A. Mario Marcondes is also supported by a grant from the J.P. McCarthy Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marcondes, A.M., Bair, S., Rabinovitch, P.S. et al. No telomere shortening in marrow stroma from patients with MDS. Ann Hematol 88, 623–628 (2009). https://doi.org/10.1007/s00277-008-0649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0649-7