Abstract
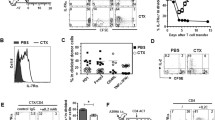
Treatment of cancer with cytotoxic agents may induce lymphopenia. Adoptively transferred T cells have been reported to display enhanced anti-tumor efficacy in the lymphopenic setting. We reasoned that the anti-tumor effects of adoptively transferred cells in the lymphopenic host could be further augmented through local provision of an innate stimulus in the tumor bed. Utilizing a model in which mice were irradiated to induce lymphopenia, with limited shielding to allow tumor growth, we demonstrate that “triple” therapy consisting of radiation-induced lymphopenia, adoptive transfer of naïve CD8+ T cells, and intra-tumoral HSV amplicon injection resulted in reduced tumor growth compared to the combination of any two of the aforementioned interventions. To gain insight into the mechanism underlying this effect we studied the effects of HSV amplicon transduction into tumors on cytokine expression and on anti-tumor specific T cells. HSV amplicon transduction specifically induced several cytokine mRNAs including IFN-γ, and IP-10. Adoptively transferred transgenic OT-1 T cells directed against Ovalbumin were more effective against Ovalbumin-expressing tumors when combined with intra-tumoral HSV amplicon injections in the lymphopenic host. Following intra-tumoral HSV-amplicon injections, anti-tumor T cells secreted higher levels of interferon-γ in response to in-vitro re-stimulation with tumor cells, implying that HSV amplicon injection provided a strong signal for T cell activation. Combining adoptive transfer of naïve T cells in the lymphopenic setting with local T cell stimulation may facilitate expansion and activation of anti-tumor T cell populations in vivo, resulting in enhanced anti-tumor responses without the need to resort to prolonged in vitro T cell culture and/or manipulation.







Similar content being viewed by others
References
Lonsdorf AS, Kuekrek H, Stern BV, Boehm BO, Lehmann PV, Tary-Lehmann M (2003) Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J Immunol 171:3941–3946
Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, Weiner GJ (2001) Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol 167:4878–4886
Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298:850–854
Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA et al (2005) Adoptive cell transfer therapy following non-myeloablative but lympho-depleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23:2346–2357
Dudley ME, Rosenberg SA (2003) Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 3:666–675
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6:345–352
Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T (1998) B cells inhibit induction of T cell-dependent tumor immunity. Nat Med 4:627–630
Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH et al (2005) Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer 117:574–586
Goldrath AW, Bogatzki LY, Bevan MJ (2000) Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med 192:557–564
Ge Q, Hu H, Eisen HN, Chen J (2002) Naive to memory T-cell differentiation during homeostasis-driven proliferation. Microbes Infect 4:555–558
Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D (2004) The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci USA 101:16885–16890
Tanchot C, Le Campion A, Martin B, Leaument S, Dautigny N, Lucas B (2002) Conversion of naive T cells to a memory-like phenotype in lymphopenic hosts is not related to a homeostatic mechanism that fills the peripheral naive T cell pool. J Immunol 168:5042–5046
Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, Chang AE (2003) Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res 63:8466–8475
Cao ZA, Daniel D, Hanahan D (2002) Sub-lethal radiation enhances anti-tumor immunotherapy in a transgenic mouse model of pancreatic cancer. BMC Cancer 2:11
Maine GN, Mule JJ (2002) Making room for T cells. J Clin Invest 110:157–159
Asavaroengchai W, Kotera Y, Mule JJ (2002) Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci USA 99:931–936
Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN (2002) T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest 110:185–192
Hu HM, Poehlein CH, Urba WJ, Fox BA (2002) Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res 62:3914–3919
Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM (2003) Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol 33:2123–2132
Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR (1994) T cell receptor antagonist peptides induce positive selection. Cell 76:17–27
Ikawa M, Yamada S, Nakanishi T, Okabe M (1998) ‘Green mice’ and their potential usage in biological research. FEBS Lett 430:83–87
Geller AI, Freese A (1990) Infection of cultured central nervous system neurons with a defective herpes simplex virus 1 vector results in stable expression of Escherichia coli beta-galactosidase. Proc Natl Acad Sci USA 87:1149–1153
Bowers WJ, Olschowka JA, Federoff HJ (2003) Immune responses to replication-defective HSV-1 type vectors within the CNS: implications for gene therapy. Gene Ther 10:941–945
Dhanji S, Teh SJ, Oble D, Priatel JJ, Teh HS (2004) Self-reactive memory-phenotype CD8 T cells exhibit both MHC-restricted and non-MHC-restricted cytotoxicity: a role for the T-cell receptor and natural killer cell receptors. Blood 104:2116–2123
Cristofanilli M, Krishnamurthy S, Guerra L, Broglio K, Arun B, Booser DJ, Menander K, Van Wart Hood J, Valero V, Hortobagyi GN (2006) A nonreplicating adenoviral vector that contains the wild-type p53 transgene combined with chemotherapy for primary breast cancer: safety, efficacy, and biologic activity of a novel gene-therapy approach. Cancer 107:935–944
Strbo N, Oizumi S, Sotosek-Tokmadzic V, Podack ER (2003) Perforin is required for innate and adaptive immunity induced by heat shock protein gp96. Immunity 18:381–390
el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, Feldman M, Eisenbach L (1999) MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol 29:3295–3301
Kutubuddin M, Federoff HJ, Challita-Eid PM, Halterman M, Day B, Atkinson M, Planelles V, Rosenblatt JD (1999) Eradication of pre-established lymphoma using herpes simplex virus amplicon vectors. Blood 93:643–654
Tolba KA, Bowers WJ, Muller J, Housekneckt V, Giuliano RE, Federoff HJ, Rosenblatt JD (2002) Herpes simplex virus (HSV) amplicon-mediated codelivery of secondary lymphoid tissue chemokine and CD40L results in augmented antitumor activity. Cancer Res 62:6545–6551
Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW (2004) The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol 173:1738–1743
Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ (2001) Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol 166:1097–1105
Curtsinger JM, Lins DC, Mescher MF (2003) Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med 197:1141–1151
Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF (1999) Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 162:3256–3262
Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF (2005) Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 174:4465–4469
CpG 7909: PF 3512676, PF-3512676. Drugs 2006 R D, 7:312–316
Acknowledgments
The authors would like to thank Mr. Wade Narrow for preparation of HSV amplicon vector stocks. Supported by 5-R01-CA-87978-06 (P.I. Joseph Rosenblatt, Co-P.I. Khaled A. Tolba) NIH/NCI Title: HSV amplicon activation of innate and adaptive immunity. D. M. was supported by an ASCO Young Investigator Award. K. T. was supported by an American Cancer Society Institutional Research Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nechushtan, H., Pham, D., Zhang, Y. et al. Augmentation of anti-tumor responses of adoptively transferred CD8+T cells in the lymphopenic setting by HSV amplicon transduction. Cancer Immunol Immunother 57, 663–675 (2008). https://doi.org/10.1007/s00262-007-0405-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0405-1