Abstract
Purpose
Complementing clinical findings with those generated by biomarkers—such as β-amyloid-targeted positron emission tomography (PET) imaging—has been proposed as a means of increasing overall accuracy in the diagnosis of Alzheimer’s disease (AD). Florbetaben ([18F]BAY 94-9172) is a novel β-amyloid PET tracer currently in global clinical development. We present the results of a proof of mechanism study in which the diagnostic efficacy, pharmacokinetics, safety and tolerability of florbetaben were assessed. The value of various quantitative parameters derived from the PET scans as potential surrogate markers of cognitive decline was also investigated.
Methods
Ten patients with mild-moderate probable AD (DSM-IV and NINCDS-ADRDA criteria) and ten age-matched (≥ 55 years) healthy controls (HCs) were administered a single dose of 300 MBq florbetaben, which contained a tracer mass dose of < 5 μg. The 70–90 min post-injection brain PET data were visually analysed by three blinded experts. Quantitative assessment was also performed via MRI-based, anatomical sampling of predefined volumes of interest (VOI) and subsequent calculation of standardized uptake value (SUV) ratios (SUVRs, cerebellar cortex as reference region). Furthermore, single-case, voxelwise analysis was used to calculate individual “whole brain β-amyloid load”.
Results
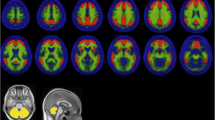
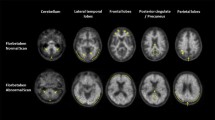
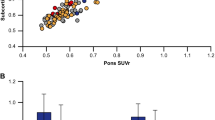
Visual analysis of the PET data revealed nine of the ten AD, but only one of the ten HC brains to be β-amyloid positive (p = 0.001), with high inter-reader agreement (weighted kappa ≥ 0.88). When compared to HCs, the neocortical SUVRs were significantly higher in the ADs (with descending order of effect size) in frontal cortex, lateral temporal cortex, occipital cortex, anterior and posterior cingulate cortices, and parietal cortex (p = 0.003–0.010). Voxel-based group comparison confirmed these differences. Amongst the PET-derived parameters, the Statistical Parametric Mapping-based whole brain β-amyloid load yielded the closest correlation with the Mini-Mental State Examination scores (r = −0.736, p < 0.001), following a nonlinear regression curve. No serious adverse events or other safety concerns were seen.
Conclusion
These results indicate florbetaben to be a safe and efficacious β-amyloid-targeted tracer with favourable brain kinetics. Subjects with AD could be easily differentiated from HCs by both visual and quantitative assessment of the PET data. The operator-independent, voxel-based analysis yielded whole brain β-amyloid load which appeared valuable as a surrogate marker of disease severity.






Similar content being viewed by others
References
Tarditi A, Caricasole A, Terstappen G. Therapeutic targets for Alzheimer’s disease. Expert Opin Ther Targets 2009;13:551–67.
Bergmans BA, De Strooper B. Gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol 2010;9:215–26.
Taupin P. Adult neurogenesis, neural stem cells and Alzheimer’s disease: developments, limitations, problems and promises. Curr Alzheimer Res 2009;6:461–70.
Alzheimer’s Disease International. World Alzheimer Report 2009. http://www.alz.co.uk/research/worldreport.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59.
Gauthier S, Scheltens P. Can we do better in developing new drugs for Alzheimer’s disease? Alzheimers Dement 2009;5:489–91.
Pike KE, Savage G. Memory profiling in mild cognitive impairment: can we determine risk for Alzheimer’s disease? J Neuropsychol 2008;2:361–72.
Kukull WA, Larson EB, Reifler BV, Lampe TH, Yerby MS, Hughes JP. The validity of 3 clinical diagnostic criteria for Alzheimer’s disease. Neurology 1990;40:1364–9.
Jellinger K, Danielczyk W, Fischer P, Gabriel E. Clinicopathological analysis of dementia disorders in the elderly. J Neurol Sci 1990;95:239–58.
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–46.
Jack Jr CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010;9:119–28.
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 1994. p. 143–7. DSM-IV.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44.
Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull 1988;24:641–52.
Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc 1989;37:730–4.
Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl Med Biol 2005;32:799–809.
Patt M, Schildan A, Barthel H, Becker G, Schultze-Mosgau MH, Rohde B, et al. Metabolite analysis of [18F]florbetaben (BAY 94-9172) in human subjects: a substudy within a proof of mechanism clinical trial. J Radioanaly Nucl Chem 2010;284:557–62.
Meyer PM, Strecker K, Kendziorra K, Becker G, Hesse S, Woelpl D, et al. Reduced α4β2*-nicotinic acetylcholine receptor binding and its relationship to mild cognitive and depressive symptoms in Parkinson disease. Arch Gen Psychiatry 2009;66:866–77.
Svedberg MM, Hall H, Hellström-Lindahl E, Estrada S, Guan Z, Nordberg A, et al. [(11)C]PIB-amyloid binding and levels of Abeta40 and Abeta42 in postmortem brain tissue from Alzheimer patients. Neurochem Int 2009;54:347–57.
Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 2008;7:129–35.
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988.
Haylett DG. Textbook of receptor pharmacology. Boca Raton: CRC; 2002.
Cicchetti DV, Allison T. New procedure for assessing reliability of scoring EEG sleep recordings. Am J EEG Technol 1971;11:101–9.
Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s disease. Proc Natl Acad Sci U S A 2010;107:5949–54.
Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Någren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 2009;73:754–60.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–19.
Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007;68:1205–12.
Thompson PW, Lockhart A. Monitoring the amyloid beta-peptide in vivo—caveat emptor. Drug Discov Today 2009;14:241–51.
Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 2010;9:363–72.
O’Keefe GJ, Saunder TH, Ng S, Ackerman U, Tochon-Danguy HJ, Chan JG, et al. Radiation dosimetry of beta-amyloid tracers 11C-PiB and 18F-BAY94-9172. J Nucl Med 2009;50:309–15.
Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron 2009;63:287–303.
Morris CJ, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–31.
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–56.
Saunders AM, Schmader K, Breitner JC, Benson MD, Brown WT, Goldfarb L, et al. Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet 1993;342:710–1.
Drzezga A, Grimmer T, Henriksen G, Mühlau M, Perneczky R, Miederer I, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology 2009;72:1487–94.
Stankoff B, Freeman L, Aigrot MS, Chardain A, Dollé F, Williams A, et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-(11) C]-2-(4'-methylaminophenyl)-6-hydroxybenzothiazole. Ann Neurol 2010 [Epub ahead of print].
Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–52.
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68:1718–25.
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65:1509–17.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45:358–68.
Scheinin NM, Aalto S, Koikkalainen J, Lötjönen J, Karrasch M, Kemppainen N, et al. Follow-up of [11C]PIB uptake and brain volume in patients with Alzheimer disease and controls. Neurology 2009;73:1186–92.
Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 2007;130:2837–44.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–47.
Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005;46:1959–72.
Furst AJ, Rabinovici GD, Rostomian AH, Steed T, Alkalay A, Racine C, et al. Cognition, glucose metabolism and amyloid burden in Alzheimer’s disease. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.03.011.
Harvan JR, Cotter V. An evaluation of dementia screening in the primary care setting. J Am Acad Nurse Pract 2006;18:351–60.
Acknowledgements
We would like to thank all patients, their caregivers, and the healthy volunteers who participated in this trial. Further, the support of the PET and cyclotron teams of the Department of Nuclear Medicine, University of Leipzig, is greatly acknowledged. This trial was supported by Bayer Healthcare (Berlin, Germany).
Conflicts of interest
HB, BS, and OS receive consultant honoraria from Bayer Healthcare. TZ, JR, BR, and CR are employees of Bayer Healthcare. The other authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barthel, H., Luthardt, J., Becker, G. et al. Individualized quantification of brain β-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer’s disease and healthy controls. Eur J Nucl Med Mol Imaging 38, 1702–1714 (2011). https://doi.org/10.1007/s00259-011-1821-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1821-1