Abstract
Purpose
Radioimmunotherapy (RIT) using 131I-3F8 injected into cerebrospinal fluid (CSF) was a safe modality for the treatment of leptomeningeal metastases (JCO, 25:5465, 2007). A single-compartment pharmacokinetic model described previously (JNM 50:1324, 2009) showed good fitting to the CSF radioactivity data obtained from patients. We now describe a two-compartment model to account for the ventricular reservoir of 131I-3F8 and to identify limiting factors that may impact therapeutic ratio.
Methods
Each parameter was examined for its effects on (1) the area under the radioactivity concentration curve of the bound antibody (AUC[CIAR]), (2) that of the unbound antibody AUC[CIA], and (3) their therapeutic ratio (AUC[CIAR]/AUC[CIA]).
Results
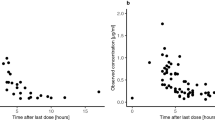
Data fitting showed that CSF kBq/ml data fitted well using the two-compartment model (R = 0.95 ± 0.03). Correlations were substantially better when compared to the one-compartment model (R = 0.92 ± 0.11 versus 0.77 ± 0.21, p = 0.005). In addition, we made the following new predictions: (1) Increasing immunoreactivity of 131I-3F8 from 10% to 90% increased both (AUC[CIAR]) and therapeutic ratio ([AUC[CIAR]/AUC[CIA]] by 7.4 fold, (2) When extrapolated to the clinical setting, the model predicted that if 131I-3F8 could be split into 4 doses of 1.4 mg each and given at ≥24 hours apart, an antibody affinity of KD of 4 × 10-9 at 50% immunoreactivity were adequate in order to deliver ≥100 Gy to tumor cells while keeping normal CSF exposure to <10 Gy.
Conclusions
This model predicted that immunoreactivity, affinity and optimal scheduling of antibody injections were crucial in improving therapeutic index.





Similar content being viewed by others
References
Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5:443–52.
Bruno MK, Raizer J. Leptomeningeal metastases from solid tumors (meningeal carcinomatosis). Cancer Treat Res. 2005;125:31–52.
Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–19.
Smith DB, Howell A, Harris M. Carcinomatous meningitis associated with infiltrating lobular carcinoma of the breast. European Journal Surgical Oncology. 1985;11:33–6.
Posner JB. Neurologic Complications of Cancer, Comtemporary neurology series. Philadelphia: F.A. Davis Company; 1995.
Kramer K, Kushner B, Heller G, Cheung NK. Neuroblastoma metastatic to the central nervous system. The Memorial Sloan-kettering Cancer Center Experience and A Literature Review. Cancer. 2001;91:1510–9.
Shaw PJ. Neuroblastoma with intracranial involvement: an ENSG study. Medical Pediatric Oncology. 1992;20:149–55.
Kellie JJ, Hayes A, Bowman L, et al. Primary extracranial neuroblastoma with central nerbous system metastases: characterization by clinicopathologic findings and neuroimaging. Cancer. 1991;68:1999–2006.
Moseley RP, Davies AG, Richardson RB, et al. Intrathecal administration of I-131 radiolabelled monoclonal antibody as a treatment for neoplastic meningitis. Br J Cancer. 1990;62:637–42.
Bigner DD, Brown M, Coleman E, et al. Phase I studies of treatment of malignant gliomas and neoplastic meningitis with 131 I radiolabeled monoclonal antibodies anti-tenascin 81C6 and anti-chondroitin proteoglycan sulfate Mel-14 (ab')2 - a preliminary report. J Neuro-Oncol. 1995;24:109–22.
Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25:5465–70.
Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol 2009.
Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvascular research. 1989;37:77–104.
Eger RR, Covell DG, Carrasquillo JA, et al. Kinetic model for the biodistribution of an 111In-labeled monoclonal antibody in humans. Cancer Res. 1987;47:3328–36.
Thierens HM, Monsieurs MA, Brans B, Van Driessche T, Christiaens I, Dierckx RA. Dosimetry from organ to cellular dimensions. Comput Med Imaging Graph. 2001;25:187–93.
Lv Y, Cheung NK, Fu BM. A pharmacokinetic model for radioimmunotherapy delivered through cerebrospinal fluid for the treatment of leptomeningeal metastases. J Nucl Med. 2009;50:1324–31.
Brassow F, Baumann K. Volume of brain ventricles in man determined by computer tomography. Neuroradiology. 1978;16:187–9.
Silverberg GD, Heit G, Huhn S, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer's type. Neurology. 2001;57:1763–6.
Guyton A, Hall J. Textbook of medical physiology. Philadelphia: Saunders; 2000.
Blasberg RG, Patlak CS, Shapiro WR. Distribution of methotrexate in the cerebrospinal fluid and brain after intraventricular administration. Cancer treatment reports. 1977;61:633–41.
Davson H, Hollingsworth G, Segal MB. The mechanism of drainage of the cerebrospinal fluid. Brain. 1970;93:665–78.
Yeh SD, Larson SM, Burch L, et al. Radioimmunodetection of neuroblastoma with iodine-131-3F8: Correlation with biopsy, iodine-131-metaiodobenzylguanidine (MIBG) and standard diagnostic modalities. J Nucl Med. 1991;32:769–76.
Koppe MJ, Hendriks T, Boerman OC, Oyen WJ, Bleichrodt RP. Radioimmunotherapy is an effective adjuvant treatment after cytoreductive surgery of experimental colonic peritoneal carcinomatosis. J Nucl Med. 2006;47:1867–74.
Kukis DL, DeNardo SJ, DeNardo GL, O'Donnell RT, Meares CF. Optimized conditions for chelation of yttrium-90-DOTA immunoconjugates. J Nucl Med. 1998;39:2105–10.
Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest. 2007;25:67–77.
Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45:2642–9.
Mujoo K, Kipps TJ, Yang HM, et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–61.
Gillies SD, Lo KM, Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods. 1989;125:191–202.
Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the children's oncology group. J Clin Oncol 2008.
FDA US. Radiation Quantities and Units. In: Department of Health and Human Services 2009.
Acknowledgements
Supported in part by grants from the National Institutes of Health CA106450, Robert Steel Foundation, Hope Street Kids, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, Ludwig Center for Cancer Immunotherapy, and the Experimental Research Center of MSKCC. We want to thank Dr. Bingmei Fu of City College of the City University of New York for her critical review of the manuscript. Our thanks to Drs. Jorge Carasquillo and Neeta Pandit-Taskar of MSKCC for their administration of intraOmmaya 131I-3F8, and to Dr. Irene Cheung for critical review of the manuscript. N-K.V. Cheung was named as the inventor of antibody 3F8, which was assigned to Memorial-Sloan Kettering Cancer Center. 3F8 was licensed by Memorial-Sloan Kettering Cancer Center to United Therapeutics, Inc. United Therapeutics did not sponsor any of the laboratory or clinical research reported in these studies. The other authors disclosed no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
U.S. Department of Health and Human Services. Radiation Quantities and Units. July 27, 2009 [cited 2009 August 24]; Available from: ≤http://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115335.htm≥
An erratum to this article can be found at http://dx.doi.org/10.1007/s00259-010-1653-4
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 312 kb)
Rights and permissions
About this article
Cite this article
He, P., Kramer, K., Smith-Jones, P. et al. Two-compartment model of radioimmunotherapy delivered through cerebrospinal fluid. Eur J Nucl Med Mol Imaging 38, 334–342 (2011). https://doi.org/10.1007/s00259-010-1633-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1633-8