Abstract
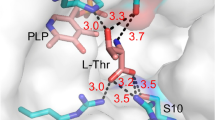
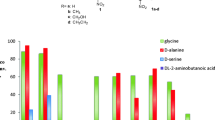
Low-specificity l-threonine aldolase, catalyzing the reversible cleavage/condensation reaction between l-threonine/l-allo-threonine and glycine plus acetaldehyde, was purified to homogeneity from Pseudomonas sp. NCIMB 10558. The enzyme has an apparent molecular mass of approximately 145 kDa and consists of four identical subunits with a molecular mass of 38 kDa. The enzyme, requiring pyridoxal- 5′-phosphate as a coenzyme, is strictly l-specific at the α position, whereas it can not distinguish between threo and erythro forms at the β position. Besides the reversible cleavage/condensation of threonine, the enzyme also catalyzes the reversible interconversion between glycine plus various aldehydes and l-β-hydroxy-α-amino acids, including l-β-(3,4-dihydroxyphenyl)serine, l-β-(3,4-met‐hylenedioxyphenyl)serine and l-β-phenylserine, providing a new route for the industrial production of these important amino acids.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 10 November 1997 / Received revision: 7 January 1998 / Accepted 30 January 1998
Rights and permissions
About this article
Cite this article
Liu, J., Ito, S., Dairi, T. et al. Low-specificity l-threonine aldolase of Pseudomonas sp. NCIMB 10558: purification, characterization and its application to β-hydroxy-α-amino acid synthesis. Appl Microbiol Biotechnol 49, 702–708 (1998). https://doi.org/10.1007/s002530051235
Issue Date:
DOI: https://doi.org/10.1007/s002530051235