Abstract
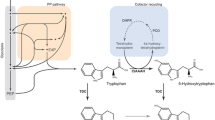
Serotonin derivatives belong to a class of phenylpropanoid amides found at low levels in a wide range of plant species. Representative serotonin derivatives include feruloylserotonin (FS) and 4-coumaroylserotonin (CS). Since the first identification of serotonin derivatives in safflower seeds, their occurrence, biological significance, and pharmacological properties have been reported. Recently, serotonin N-hydroxycinnamoyl transferase (SHT), which is responsible for the synthesis of serotonin derivatives, was cloned from pepper (Capsicum annuum) and characterized in terms of its enzyme kinetics. Using the SHT gene, many attempts have been made to either increase the level of serotonin derivatives in transgenic plants or produce serotonin derivatives de novo in microbes by dual expression of key genes such as SHT and 4-coumarate-CoA ligase (4CL). Due to the strong antioxidant activity and other therapeutic properties of serotonin derivatives, these compounds may have high potential in treatment and prophylaxis, as cosmetic ingredients, and as major components of functional foods or feeds that have health-improving effects. This review examines the biosynthesis of serotonin derivatives, corresponding enzymes, heterologous production in plants or microbes, and their applications.


Similar content being viewed by others
References
Choi SW, Park RW, Lee WJ (2002) Novel use of polyphenol compounds isolated from safflower (Carthamus tincorious L.) seeds. Korea patent 10-0354791-0000
Ehlting J, Büttner D, Wang Q, Douglas CJ, Somssich IE, Kombrink E (1999) Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J 19:9–20
Facchini PJ, Hagel J, Zulak KG (2002) Hydroxycinnamic acid amide metabolism: physiology and biochemistry. Can J Bot 80:577–589
Geerlings A, Redondo FJ, Contin A, Memelink J, van der Heijden R, Verpoorte R (2001) Biotransformation of tryptamine and secologanin into plant terpenoid indole alkaloids by transgenic yeast. Appl Microbiol Biotechnol 56:420–424
Guillet G, De Luca V (2005) Wound-inducible biosynthesis of phytoalexin hydroxycinnamic acid amides of tyramine in tryptophan and tyrosine decarboxylase transgenic tobacco lines. Plant Physiol 137:692–699
Hagel JM, Facchini PJ (2005) Elevated tyrosine decarboxylase and tyramine hydroxycinnamoyltransferase levels increase wound-induced tyramine-derived hydroxycinnamic acid amide accumulation in transgenic tobacco leaves. Planta 221:904–914
Hotta Y, Nagatsu A, Liu W, Muto T, Narumiya C, Lu X, Yajima M, Ishikawa N, Miyazeki K, Kawai N, Mizukami H, Sakakibara J (2002) Protective effects of antioxidative serotonin derivatives isolated from safflower against postischemic myocardial dysfunction. Mol Cell Biochem 238:151–162
Ishihara A, Kawata N, Matsukawa T, Iwamura H (2000) Induction of N-hydroxycinnamoyltyramine synthesis and tyramine N-hydroxycinnamoyltransferase (THT) activity by wounding in maize leaves. Biosci Biotechnol Biochem 64:1025–1031
Ishihara A, Hashimoto Y, Tanaka C, Dubouzet JG, Nakao T, Matsuda F, Nishioka T, Miyagawa H, Wakasa K (2008) The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J 54:481–495
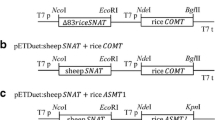
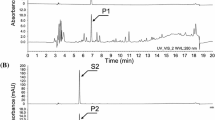
Jang SM, Ishihara A, Back K (2004) Production of coumaroylserotonin and feruloylserotonin in transgenic rice expressing pepper hydroxycinnamoyl-coenzyme A:serotonin N-(hydroxycinnamoyl) transferase. Plant Physiol 135:346–356
Jenett-Siems K, Weigl R, Kaloga M, Schulz J, Eich E (2003) Ipobscurines C and D: macrolactam-type indole alkaloids from the seeds of Ipomoea obscura. Phytochemistry 62:1257–1263
Kang S, Back K (2006) Enriched production of N-hydroxycinnamic acid amides and biogenic amines in pepper (Capsicum annuum) flowers. Sci Hortic 108:337–341
Kang K, Back K (2009) Production of phenylpropanoid amides in recombinant Escherichia coli. Metab Eng 11:64–68
Kang K, Jang SM, Kang S, Back K (2005) Enhanced neutraceutical serotonin derivatives of rice seed by hydroxycinnamoyl-CoA:serotonin N-(hydroxycinnamoyl) transferase. Plant Sci 168:783–788
Kang S, Kang K, Chung GC, Choi D, Ishihara A, Lee DS, Back K (2006) Functional analysis of the amine substrate specificity domain of pepper tyramine and serotonin N-hydroxycinnamoyltransferases. Plant Physiol 140:704–715
Kang S, Kang K, Lee K, Back K (2007a) Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227:263–272
Kang S, Kang K, Lee K, Back K (2007b) Characterization of tryptamine 5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep 26:2009–2015
Kang K, Lee K, Sohn SO, Park S, Lee S, Kim SY, Kim YS, Back K (2009) Ectopic expression of serotonin N-hydroxycinnamoyltransferase and different production of phenylpropanoid amides in transgenic tomato tissues. Sci Hortic doi: 1016/j.scienta.2008.12.015
Koyama N, Kuribayashi K, Seki T, Kobayashi K, Furuhata Y, Suzuki K, Arisaka H, Nakano T, Amino Y, Ishii K (2006) Serotonin derivatives, major safflower (Carthamus tinctorius L.) seed antioxidants, inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem 54:4970–4976
Koyama N, Kuribayashi K, Ishii K, Kobayashi K (2009) Composition for preventing atherosclerosis. US patent 07,485,328
Kumarasamy Y, Middleton M, Reid RG, Nahar L, Sarker SD (2003) Biological activity of serotonin conjugates from the seeds of Centaurea nigra. Fitoterapia 74:609–612
Lee DG, Park Y, Kim MR, Jung HJ, Seu YB, Hahm KS, Woo ER (2004) Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol Lett 26:1125–130
Lee K, Kang K, Park M, Woo YM, Back K (2008) Endosperm-specific expression of serotonin N-hydroxycinnamoyltransferase in rice. Plant Foods Hum Nutr 63:53–57
Ly D, Kang K, Choi JY, Ishihara A, Back K, Lee SG (2008) HPLC analysis of serotonin, tryptamine, tyramine, and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J Med Food 11:385–389
Martin-Tanguy J (1985) The occurrence and possible function of hydroxycinnamoyl acid amides in plants. Plant Growth Regul 3:381–399
Mijts BN, Schmidt-Dannert C (2003) Engineering of secondary metabolite pathways. Curr Opin Biotechnol 14:597–602
Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep 19:698–704
Nagatsu A, Zhang HL, Mizukami H, Okuyama H, Sakakibara J, Tokuda H, Nishino H (2000) Tyrosinase inhibitory and anti-tumor promoting activities of compounds isolated from safflower (Carthamus tinctorius L.) and cotton (Gossypium hirsutum L.) oil cakes. Nat Prod Lett 14:153–158
Niwa T, Etoh H, Shimizu A, Shimizu Y (2000) Cis-N-(p-coumaroyl) serotonin from konnyaku, Amorphophallus konjac K. Koch. Biosci Biotechnol Biochem 64:2269–2271
Noé W, Mollenschott C, Berlin J (1984) Tryptophan decarboxylase from Catharanthus roseus cell suspension cultures: purification, molecular and kinetic data of the homogenous protein. Plant Mol Biol 3:281–288
Park JB (2008) Serotomide and safflomide modulate forskolin-stimulated cAMP formation via 5-HT1 receptor. Phytomedicine 15:1093–1098
Park JB, Schoene N (2002) Synthesis and characterization of N-coumaroyltyramine as a potent phytochemical which arrests human transformed cells via inhibiting protein tyrosine kinases. Biochem Biophys Res Commun 292:1104–1110
Park M, Kang K, Park S, Back K (2008a) Conversion of 5-hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli, and yeast. Biosci Biotechnol Biochem 72:2456–2458
Park M, Kang K, Park S, Kim YS, Ha SH, Lee SW, Ahn MJ, Bae JM, Back K (2008b) Expression of serotonin derivative synthetic genes on a single self-processing polypeptide and the production of serotonin derivatives in microbes. Appl Microbiol Biotechnol 81:43–49
Pavlík M, Laudová V, Grüner K, Vokáč K, Harmatha J (2002) High-performance liquid chromatographic analysis and separation of N-feruloylserotonin isomers. J Chromatogr 770:291–295
RadWanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921–934
Roh JS, Han JY, Kim JH, Hwang JK (2004) Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol Pharm Bull 27:1976–1978
Ryan MD, King AMQ, Thomas GP (1991) Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol 72:2727–2732
Sakamura S, Terayama Y, Kawakatsu S, Ichihara A, Saito H (1978) Conjugated serotonins related to cathartic activity in safflower seed (Carthamus tinctorius L.). Agric Biol Chem 42:1805–1806
Sarker SD, Laird A, Nahar L, Kumarasamy Y, Jaspars M (2001) Indole alkaloids from the seeds of Centaurea cyanus (Asteraceae). Phytochemistry 57:1273–1276
Schröder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W (1999) Latest on the enzymology of serotonin biosynthesis in walnut seeds. Adv Exp Med Biol 467:637–644
Shoeb M, MacManus S, Jaspars M, Trevidu J, Nahar L, Kong-Thoo-Lin P, Sarker SD (2006) Montamine, a unique dimeric indole alkaloid, from the seeds of Centaurea montana (Asteraceae), and its in vitro cytotoxic activity against the CaCo2 colon cancer cells. Tetrahedron 62:11172–11177
Takii T, Hayashi M, Hiroma H, Chiba T, Kawashima S, Zhang HL, Nagatsu A, Sakakibara J, Onozaki K (1999) Serotonin derivative, N-(p-coumaroyl) serotonin, isolated from safflower (Carthamus tinctorius L.) oil cake augments the proliferation of normal human and mouse fibroblasts in synergy with basic fibroblast growth factor (bFGF) or epidermal growth factor (EGF). J Biochem 125:910–915
Takii T, Kawashima S, Chiba T, Hayashi H, Hayashi M, Hiroma H, Kimura H, Inukai Y, Shibata Y, Nagatsu A, Sakakibara J, Oomoto Y, Hirose K, Onozaki K (2003) Multiple mechanisms involved in the inhibition of proinflammatory cytokine production from human monocytes by N-(p-coumaroyl) serotonin and its derivatives. Immunopharmacology 3:273–277
Tanaka E, Tanaka C, Mori N, Kuwahara Y, Tsuda M (2003) Phenylpropanoid amides of serotonin accumulate in witchs’ broom diseased bamboo. Phytochemistry 64:965–969
Tozawa Y, Hasegawa H, Teruhiko T, Wakasa K (2001) Characterization of rice anthranilate synthase α-subunit genes OASA1 and OASA2. Tryptophan accumulation in transgenic rice expressing a feedback-insensitive mutant OASA1. Plant Physiol 126:1493–1506
Watanabe M (1999) Antioxidative phenolic compounds from Japanese barnyard millet (Echinochloa utilis) grains. J Agric Food Chem 47:4500–4505
Wink M (1997) Special nitrogen metabolism. In: Dey PM, Harborne JB (eds) Plant Biochemistry. Academic, San Diego, pp 439–486
Yamamotová A, Pometlova M, Harmatha J, Raskova H, Rokyta R (2007) The selective effect of N-feruloylserotonins isolated from Leuzea carthamoides on nociception and anxiety in rats. J Ethnopharm 112:368–374
Yuji N, Naoto K, Katsuya S, Hideaki K, Yuka I (2007) Anti-inflammatory composition. PCT patent 2007129743
Zhang HL, Nagatsu A, Sakakibara J (1996) Novel antioxidants from safflower (Carthamus tinctorius L.) oil cake. Chem Pharm Bull 44:874–876
Acknowledgments
Thanks are expressed to Prof. A. Ishihara of the Graduate School of Kyoto University (Kyoto, Japan) for technical support. This work was supported by the Technology Development Program for Agriculture and Forestry, Ministry of Agriculture, Forestry, and Fisheries, and by the SRC program of MOST/KOSEF, through the Agricultural Plant Stress Research Center (R11-2001-092-05001-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, K., Park, S., Kim, Y.S. et al. Biosynthesis and biotechnological production of serotonin derivatives. Appl Microbiol Biotechnol 83, 27–34 (2009). https://doi.org/10.1007/s00253-009-1956-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1956-1