Abstract
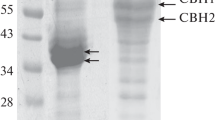
Two extracellular tannin acyl hydrolases (TAH I and TAH II) produced by an Antarctic filamentous fungus Verticillium sp. P9 were purified to homogeneity (7.9- and 10.5-fold with a yield of 1.6 and 0.9%, respectively) and characterized. TAH I and TAH II are multimeric (each consisting of approximately 40 and 46 kDa sub-units) glycoproteins containing 11 and 26% carbohydrates, respectively, and their molecular mass is approximately 155 kDa. TAH I and TAH II are optimally active at pH of 5.5 and 25 and 20°C, respectively. Both the enzymes were activated by Mg2+and Br− ions and 0.5–2.0 M urea and inhibited by other metal ions (Zn2+, Cu2+, K+, Cd2+, Ag+, Fe3+, Mn2+, Co2+, Hg2+, Pb2+ and Sn2+),\({\text{CO}}_{\text{3}}^{{\text{2}} - }\) anions, Tween 20, Tween 60, Tween 80, Triton X-100, sodium dodecyl sulphate, β-mercaptoethanol, α-glutathione and 4-chloromercuribenzoate. Both tannases more efficiently hydrolyzed tannic acid than methyl gallate. E a of these reactions and temperature dependence (at 0–30°C) of k cat, k cat/K m, ΔG*, ΔH* and ΔS* for both the enzymes and substrates were determined. The k cat and k cat/K m values (for both the substrates) were considerably higher for the combined preparation of TAH I and TAH II.







Similar content being viewed by others
References
Aguilar CN, Gutiérrez-Sánchez G (2001) Review: sources, properties, application and potential uses of tannin acyl hydrolase. Food Sci Technol Int 7:373–382
Banerjee D, Mondal KC, Pati BR (2001) Production and characterization of extracellular and intracellular tannase from newly isolated Aspergillus aculeatus DBF 9. J Basic Microbiol 41:313–318
Barthomeuf C, Regerat F, Pourrat H (1994) Production, purification and characterization of a tannase from Aspergillus niger LCF 8. J Ferment Bioeng 77:320–323
Batra A, Saxena RK (2005) Potential tannase producers from the genera Aspergillus and Penicillium. Process Biochem 40:1553–1557
Battestin V, Macedo GA (2007) Effects of temperature, pH and additives on the activity of tannase produced by Paecilomyces variotii. Electr J Biotechnol 10:191–199
Bhardwaj R, Singh B, Bhat TK (2003) Purification and characterization of tannin acyl hydrolase from Aspergillus niger MTCC 2425. J Basic Microbiol 43:449–461
Bhat TK, Singh B, Sharma OP (1998) Microbial degradation of tannins—a current perspective. Biodegradation 9:343–357
Boadi DK, Neufeld RJ (2001) Encapsulation of tannase for the hydrolysis of tea tannin. Enzyme Microb Technol 28:590–595
Bradford MM (1976) A rapid and sensitive method for the quantification of milligram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci 121:404–427
Dubois MK, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric methods for determination of sugars and related substances. Anal Chem 28:350–356
Fahleson J, Hu Q, Dixelius C (2004) Phylogenetic analysis of Verticillium species based on nuclear and mitochondrial sequences. Arch Microbiol 181:435–442
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nature Rev Microbiol 1:200–208
Fenice M, Selbmann L, Zucconi L, Onofri S (1997) Production of extracellular enzymes by Antarctic fungal strains. Polar Biol 17:275–755
Haslam E, Stangroom JE (1996) The esterase and depsidase activities of tannase. Biochem J 99:28–31
Hatamoto O, Watarai T, Kikuchi M, Mizusawa K, Sekine H (1996) Cloning and sequencing of the gene encoding tannase and a structural study of the tannase subunit from Aspergillus oryzae. Gene 175:215–221
Iibuchi S, Minoda Y, Yamada K (1968) Studies on tannin acyl hydrolase of microorganisms. Part III. Purification of the enzyme and some properties of it. Agric Biol Chem 32:803–809
Kar B, Banerjee R, Bhattacharyya BC (2003) Effect of additives on the behavioral properties of tannin acyl hydrolase. Process Biochem 38:1285–1293
Laemmli UK (1971) Clevage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–684
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:215–260
Mahapatra K, Nanda RK, Bag SS, Banerjee R, Pandey A, Szakacs G (2005) Purification, characterization and same studies on secondary structure of tannase from Aspergillus awamori nakazawa. Process Biochem 40:3251–3254
Mahendran B, Raman N, Kim DJ (2006) Purification and characterization of tannase from Paecilomyces variotii: hydrolysis of tannic acid using immobilized tannase. Appl Microbiol Biotechnol 70:444–450
Mondal KC, Patil BR (2000) Studies on the extracellular tannase from newly isolated Bacillus licheniformis KBR 6. J Basic Microbiol 40:223–232
Mondal KC, Banerjee R, Patil BR (2000) Tannase production by Bacillus licheniformis. Biotechnol Lett 22:767–769
Mondal KC, Banerjee D, Banerjee R, Pati BR (2001) Production and characterization of tannase from Bacillus cereus KBR9. J Gen Appl Microbiol 47:263–267
Mukherjee G, Banerjee R (2006) Effects of temperature, pH and additives on the activity of tannase produced by a co-culture Rhizopus oryzae and Aspergillus foetidus. World J Microbiol Biotechnol 22:207–212
Nuero OM, Reyes F (2002) Enzymes for animal feeding from Penicillium chrysogenum mycelial wastes from penicillin manufacture. Lett Appl Microbiol 34:413–416
Ornstein L (1964) Disc electrophoresis. I. Background and theory. Ann NY Acad Sci 121:321–349
Rajakumar SG, Nandy SC (1983) Isolation, purification and some properties of Penicillium chrysogenum tannase. Appl Environ Microbiol 46:525–527
Ramírez-Coronel MA, Viniegra-González G, Darvill A, Augur C (2003) A novel tannase from Aspergillus niger with beta-glucosidase activity. Microbiol 149:2941–2946
Sabu A, Kiran GS, Pandey A (2005) Purification and characterization of tannin acyl hydrolase from Aspergillus niger ATCC 16620. Food Technol Biotechnol 43:133–138
Saxena S, Saxena RK (2004) Statistical optimization of tannase production from Penicillium variable using fruits (chebulic myrobalan) of Terminalia chebula. Biotechnol Appl Biochem 39:99–106
Sharma S, Bhat TK, Darwa RK (1999) Isolation, purification and properties of tannase from Aspergillus niger van Tieghem. World J Microbiol Biotechnol 15:673–677
Yamada H, Adachi O, Watanabe M, Sato N (1968) Studies of fungal tannase. Part I. Formation, purification and catalytic properties of tannase of Aspergillus flavus. Agric Biol Chem 32:1070–1078
Zhong X, Peng L, Zheng S, Sun Z, Ren Y, Dong M, Xu A (2004) Secretion, purification and characterization of a recombinant Aspergillus oryzae tannase in Pichia pastoris. Protein Expr Purif 36:165–169
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasieczka-Burnecka, M., Kuc, K., Kalinowska, H. et al. Purification and characterization of two cold-adapted extracellular tannin acyl hydrolases from an Antarctic strain Verticillium sp. P9. Appl Microbiol Biotechnol 77, 77–89 (2007). https://doi.org/10.1007/s00253-007-1124-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1124-4