Abstract
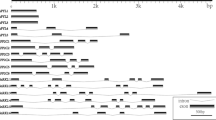
Fruit body initials of Agaricus bisporus contain high levels of urea, which decrease in the following developmental stages until stage 4 (harvest) when urea levels increase again. At storage, the high urea content may affect the quality of the mushroom, i.e. by the formation of ammonia from urea through the action of urease (EC 3.5.1.5). Despite the abundance of urea in the edible mushroom A. bisporus, little is known about its physiological role. The urease gene of A. bisporus and its promoter region were identified and cloned. The coding part of the genomic DNA was interrupted by nine introns as confirmed by cDNA analysis. The first full homobasidiomycete urease protein sequence obtained comprised 838 amino acids (molecular mass 90,694 Da, pI 5.8). An alignment with fungal, plant and bacterial ureases revealed a high conservation. The expression of the urease gene, measured by Northern analyses, was studied both during normal development of fruit bodies and during post-harvest senescence. Expression in normal development was significantly up-regulated in developmental stages 5 and 6. During post-harvest senescence, the expression of urease was mainly observed in the stipe tissue; expression decreased on the first day and remained at a basal level through the remaining sampling period.




Similar content being viewed by others
References
Baars JJP, Op den Camp HJM, Hermans JMH, Mikes V, van der Drift C, Van Griensven LJLD, Vogels GD (1994) Nitrogen assimilating enzymes in the white button mushroom Agaricus bisporus. Microbiology 140:1161–1168
Baars JJP, Op den Camp HJM, van der Drift C, Sonneberg ASM, Van Griensven LJLD (1996) A review of primary nitrogen metabolism in Agaricus bisporus. CMRI Newsl 3:1–15
Clayton CL, Pallen MJ, Kleanthous H, Wren BW, Tabaqchali S (1990) Nucleotide sequence of two genes from Helicobacter pylori encoding for urease subunits. Nucleic Acids Res 18:362–363
Collins CM, Dorazio SEF (1993) Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol Microbiol 9:907–913
Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR (2000) Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68:443–448
De Groot PWJ, Schaap PJ, Sonnenberg ASM, Visser J, Van Griensven LJLD (1996) The Agaricus bisporus hypA gene encodes a hydrophobin and specifically accumulates in peel tissue of mushroom caps during fruit body development. J Mol Biol 257:1008–1018
de Windt FE, Wagemaker MJM, Op den Camp HJM, van der Drift C (2002) Purine degradation in the edible mushroom Agaricus bisporus. Folia Microbiol 47:672–676
Donker HCW, van As H (1999) Cell water balance of white button mushrooms (Agaricus bisporus) during its post-harvest lifetime studied by quantitative magnetic resonance imaging. Biochim Biophys Acta 1427:287–297
Eastwood DC, Kingsnorth CS, Jones HE, Burton KS (2001) Genes with increased transcript levels following harvest of the sporophore of Agaricus bisporus have multiple physiological roles. Mycol Res 105:1223–1230
Ewaze JO, Moore D, Stewart GR (1978) Co-ordinate regulation of enzymes involved in ornithine metabolism and its relation to sporophore morphogenesis in Coprinus cinereus. J Gen Microbiol 107:343–357
Foret V (1990) Aspects métabolizues de la fructification d’Agaricus bisporus (Lange) Imbach. Cryptogam Mycol 11:255–287
Hammond JBW (1979) Changes in composition of harvested mushrooms (Agaricus bisporus). Phytochemistry 18:415–418
Hammond JBW, Nichols R (1979) Carbohydrate metabolism in Agaricus bisporus: changes in non-structural carbohydrates during periodic fruiting (flushing). New Phytol 83:723–730
Hausinger RP (1993) Biochemistry of nickel. Plenum, New York
Hausinger RP, Colpas GJ, Soriano A (2001) Urease: a paradigm for protein-assisted metallocenter assembly. ASM News 67:78–84
Humphrey T, Proudfoot NJ (1988) A beginning to the biochemistry of polyadenylation. Trends Genet 4:243–245
Jabri E, Karplus PA (1996) Structures of the Klebsiella aerogenes urease apoenzyme and two active-site mutants. Biochemistry 35:10616–10626
Jabri E, Carr MB, Hausinger RP, Karplus PA (1995) The crystal structure of urease from Klebsiella aerogenes. Science 286:998–1004
Kersten MASH, Müller Y, Op den Camp HJM, Vogels GD, Van Griensven LJLD, Visser J, Schaap PJ (1997) Molecular characterization of the glnA gene encoding glutamine synthetase from the edible mushroom Agaricus bisporus. Mol Gen Genet 256:179–186
Kersten MASH, Baars JJP, Op den Camp HJM, Van Griensven LJLD, van der Drift C (1998) Regulation of glutamine synthetase from the white button mushroom Agaricus bisporus. Arch Biochem Biophys 364:228–234
Kersten MASH, Müller Y, Baars JJP, Op den Camp HJM, van der Drift C, Van Griensven LJLD, Visser J, Schaap PJ (1999) NAD+-dependent glutamate dehydrogenase of the edible mushroom Agaricus bisporus: biochemical and molecular characterization. Mol Gen Genet 261:452–462
Koper TE, El-Sheikh AF, Norton JM, Klotz MG (2004) Urease-encoding genes in ammonia-oxidizing bacteria. Appl Environ Microbiol 70:2342–2348
Leftley JW, Syrett PJ (1973) Urease and ATP: urea amidolyase activity in unicellular algae. J Gen Microbiol 77:109–115
Mobley HLT, Hausinger RP (1989) Microbial urease: significance, regulation, and molecular characterization. Microbiol Rev 53:85–108
Mobley HLT, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59:451–480
Moore D, Liu M, Kuhad RC (1987a) Karyogamy-dependent enzyme derepression in the basidiomycete Coprinus. Cell Biol Int Rep 11:335–341
Moore D, Horner J, Liu M (1987b) Co-ordinate control of ammonium-scavenging enzymes in fruit body of Coprinus cinereus avoids inhibition of sporulation by ammonium. FEMS Microbiol Lett 44:239–242
Pinon R (1977) Effects of ammonium ions on sporulation of Saccharomyces cerevisiae Exp Cell Res 105:367–378
Reinbothe H, Wasternack C, Miersch J (1967) Harnstoff-Metabolismus by Basidiomyceten: IV. Untersuchungen zur Physiologie des Harnstoffs. Flora 158:27–57
Roon RJ, Levenberg B (1972) Urea amidohydrolase I. Properties of the enzyme from Candida utilis. J Biol Chem 247:4107–4113
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schaap PJ, Müller Y, Baars JJP, Op den Camp HJM, Sonnenberg ASM, Van Griensven LJLD, Visser J (1996) Nucleotide sequence and expression of the gene encoding NADP+-dependent glutamate dehydrogenase (gdhA) from Agaricus bisporus. Mol Gen Genet 250:339–347
Schaap PJ, de Groot PWJ, Müller Y, Sonnenberg ASM, Visser J (1999) Analysis of the structure of housekeeping genes of Agaricus bisporus and their spatial expression in fruit bodies. Ph.D. thesis, University of Nijmegen
Takashima K, Suga T, Mamiya G (1988) The structure of jack bean urease. The complete amino acid sequence, limited proteolysis and reactive cysteine residues. Eur J Biochem 175:151–165
Tange Y, Niwa O (1997) Identification of the ure1+ gene encoding urease in fission yeast. Curr Genet 32:244–246
Unkles SE (1992) Gene organization in industrial filamentous fungi. In: Kinghorn JR, Turner G (eds) Applied molecular genetics of filamentous fungi. Chapman & Hall, London, pp 28–53
Vogels G, van der Drift C (1976) Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev 40:403–468
Wagemaker MJM, Welboren W, van der Drift C, Jetten MSM, Van Griensven LJLD, Op den Camp HJM (2005) The ornithine cycle enzyme arginase from Agaricus bisporus and its role in urea accumulation in fruit bodies. Biochim Biophys Acta 1682:107–115
Wood DA (1985) Production and roles of extracellular enzymes during morphogenesis of basidiomycete fungi. In: Moore DM, Casselton LA, Wood DA, Frankland JC (eds) Developmental biology of higher fungi. Cambridge University Press, Cambridge, MA, pp 375–387
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Yu JJ, Smithson SL, Thomas PW, Kirkland TN, Cole GT (1997) Isolation and characterization of the urease gene (URE) from the pathogenic fungus Coccidioides immitis. Gene 198:387–391
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagemaker, M.J.M., Eastwood, D.C., van der Drift, C. et al. Expression of the urease gene of Agaricus bisporus: a tool for studying fruit body formation and post-harvest development. Appl Microbiol Biotechnol 71, 486–492 (2006). https://doi.org/10.1007/s00253-005-0185-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0185-5