Abstract
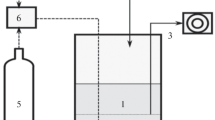
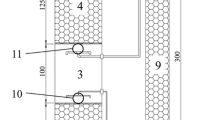
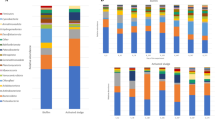
In the present work, we describe for the first time the utilization of a complex microbial biofilm for the treatment of sulfide-containing effluents. A non-aerated packed-column reactor was inoculated with anoxic lake sediment and exposed to light. A biofilm developed in the column and showed a stable oxidation performance for several weeks. Microbial species composition was analyzed by microscopy, pigment analysis and a bacterial 16S rRNA gene clone library. Colorless sulfur bacteria, green algae and purple sulfur bacteria were observed microscopically. Pigment composition confirmed the presence of algae and purple sulfur bacteria. The clone library was dominated by alpha-Proteobacteria (mostly Rhodobacter group), followed by gamma-Proteobacteria (Chromatiaceae-like and Thiothrix-like aerobic sulfur oxidizers) and the Cytophaga-Flavobacterium-Bacteroides group. Plastid signatures from algae were also present and a few clones belonged to both the beta- (Rhodoferax sp., Thiobacillus sp.) and delta-Proteobacteria (Desulfocapsa sp.) and to the low G+C Gram-positive bacteria (Firmicutes group). The coexistence of aerobic, anaerobic, phototrophic and chemotrophic microorganisms in the biofilm, the species richness found within these metabolic groups (42 operational taxonomic units) and the microdiversity observed within some species could be very important for the long-term functioning and versatility of the reactor.



Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Brock TD (1999) The genus Leucothrix. In: Dworkin T, et al (eds) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn. Springer, Berlin Heidelberg New York, http://link.springer-ny.com/link/service/books/10125/
Brown ML, Gauthier JJ (1993) Cell density and growth phase as factors in the resistance of a biofilm of Pseudomonas aeruginosa (ATCC 27853) to iodine. Appl Environ Microbiol 59:2320–2322
Brown MRW, Allison DG, Gilbert P (1988) Resilience of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother 22:777–783
Buisman CJ, Geraats BG, Ijspeert P, Lettinga G (1990) Optimization of sulphur production in a biotechnological sulphide-removing reactor. Biotechnol Bioeng 35:50–56
Canter-Lund H, Lund, JWG (1995) Freshwater algae. Their microscopic world explored. Biopress, Bristol
Casamayor EO, Schäfer H, Bañeras LL, Pedrós-Alió C, Muyzer G (2000) Identification of and spatio-temporal differences between microbial assemblages from two neighboring sulphurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl Environ Microbiol 66:499–508
Casamayor EO, Pedrós-Alió C, Muyzer G, Amann R (2002) Microheterogeneity in 16S ribosomal DNA-defined bacterial populations from a stratified planktonic environment is related to temporal changes and to ecological adaptations. Appl Environ Microbiol 68:1706–1714
Characklis WG, Wilderer PA (1989) Structure and function of biofilms. Wiley, Chichester
Cork DJ, Garunas R, Sajjad A (1983) Chlorobium limicola forma thiosulphatophilum: biocatalyst in the production of sulphur and organic carbon from a gas stream containing H2S and CO2. Appl Environ Microbiol 45:913–918
De Wit R, Van Gemerden H (1990) Growth of the photoautotrophic purple sulfur bacterium Thiocapsa roseopersicina under oxic/anoxic regimes in the light. FEMS Microbiol Ecol 73:69–76
Felske A, Akkermans ADL, Devos WM (1998) Quantification of 16S rRNAs in complex bacterial communities by multiple competitive reverse transcription PCR in temperature gradient gel electrophoresis fingerprints. Appl Environ Microbiol 64:4581–4587
Ferrera I, Sánchez O, Mas J (2004) A new non-aerated illuminated packed-column reactor for the development of sulfide-oxidizing biofilms. Appl Microbiol Biotechnol DOI 10.1007/s00253-004-01581-y
Field KG, Gordon D, Wright T, Rappe M, Urbach E, Vergin K, Giovannoni SJ (1997) Diversity and depth-specific distribution of SAR11 cluster rRNA genes from marine planktonic bacteria. Appl Environ Microbiol 63:63–70
Fischer U (1988) Sulphur in biotechnology. Bio/Technology 6B:463–496
Furhman JA, Campbell L (1998) Microbial microdiversity. Nature 393:410–411
Gray ND, Head IM (2001) Linking genetic identity and function in communities of uncultured bacteria. Environ Microbiol 3:481–492
Guerrero R, Pedrós-Alió C, Esteve I, Mas J (1987) Communities of phototrophic sulphur bacteria in lakes of the Spanish Mediterranean region. In: Lindholm T (ed) Ecology of photosynthetic prokaryotes. Åbo Academy Press, Turku, pp 125–151
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of the microbial cells. Methods in Microbiol 5B:209–344
Imhoff JF (2001a) The phototrophic alpha-proteobacteria. In: Dworkin, et al (eds) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn. Springer, Berlin Heidelberg New York, http://link.springer-ny.com/link/service/books/10125/
Imhoff JF (2001b) The phototrophic beta-Proteobacteria. In: Dworkin, et al (eds) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn. Springer, Berlin Heidelberg New York, http://link.springer-ny.com/link/service/books/10125/
Imhoff JF (2003) The Chromatiaceae. In: Dworkin, et al (eds) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn. Springer, Berlin Heidelberg New York, http://link.springer-ny.com/link/service/books/10125/
Janssen AJH, Ma SC, Lens P, Lettinga G (1997) Performance of a sulphide-oxidizing expanded-bed reactor supplied with dissolved oxygen. Biotechnol Bioeng 53:32–40
Jensen AB, Webb C (1995) Treatment of H2S-containing gases: a review of microbiological alternatives. Enzyme Microb Technol 17:2–10
Kim BW, Kim IK, Chang HN (1990) Bioconversion of hydrogen sulphide by free and immobilized cells of Chlorobium thiosulphatophylum. Biotechnol Lett 5:381–386
Kirchman DL (2002) The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol Ecol 1317:1–10
Kobayashi HA, Stenstrom M, Mah RA (1983) Use of photosynthetic bacteria for hydrogen sulphide removal from anaerobic waste treatment effluent. Water Res 17:579–587
Ludwig W, Strunk O, Klugbauer S, Klugbaur N. Weizeneger M, Neumaier J, et al (1998) Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554–568
Maidak BL, Cole JR, Lilburn TG, Parker CT Jr, Saxman PR, Strehwick JM, et al (2000) The RDP (ribosomal database project) continues. Nucleic Acids Res 28:73–174
Nübel U, Engelen B, Felske A, Snaid J, Wieshuber A, Amann RI, et al (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643
Nübel U, Garcia-Pichel F, Kuhl M, Muyzer G (1999) Quantifying microbial diversity: morphotypes, 16S rRNA genes, and carotenoids of oxygenic phototrophs in microbial mats. Appl Environ Microbiol 65:422–430
Paul JH, Myers B (1982) Fluorometric determination of DNA in aquatic microorganisms by use of Hoechst 33258. Appl Environ Microbiol 43:1393–1399
Pedrós-Alió C, Guerrero R (1993) Microbial ecology in lake Cisó. Adv Microb Ecol 13:155–209
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Ravenschlag K, Sahm K, Pernthaler J, Amann R (1999) High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol 65:3982–3989
Sánchez O, Gemerden H van, Mas J (1996) Description of a redox-controlled sulfidostat for the growth of sulfide-oxidizing phototrophs. Appl Environ Microb 62:3640–3645
Schram A, Amann R (1998) In situ structure and function analysis of biofilms. In: Märkl H, Stegmann R (eds) Technik anaerober Prozessw. DECHEMA, Frankfurt am Main, pp 45–54
Stackebrandt E, Goebel BM (1994) Taxonomic note: a place for DNA:DNA reassociation and 16S rRNA sequence analysis in the present definition in bacteriology. Int J Syst Bacteriol 44:846–849
Stal LJ, Gemerden H van, Krumbein WE (1984) The simultaneous assay of chlorophyll and bacteriochlorophyll in natural microbial communities. J Microbiol Methods 2:295–306
Sublette KL, Sylvester ND (1987) Oxidation of hydrogen sulphide by continuous cultures of Thiobacillus denitrificans. Biotechnol Bioeng 29:753–758
Takayuki E, Li N, Kawamura Y (2001) The anaerobic gram-positive cocci. In: Dworkin, et al (eds) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn. Springer, Berlin Heidelberg New York, http://link.springer-ny.com/link/service/books/10125/
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. 1. Quantitative measurements on growing cells of Chromatium okenii. Antonie Van Leeuwenhoek 30:225–238
Von Canstein H, Kelly S, Li Y, Wagner-Döbler I (2002) Species diversity improves the efficiency of mercury-reducing biofilms under changing environmental conditions. Appl Environ Microbiol 68:2829–2837
Von Wintzingerode FV, Goebel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Wagner M, Loy A (2002) Bacterial community composition and function in sewage treatment systems. Curr Opin Biotechnol 13:218–227
West NJ, Scanlan DJ (1999) Niche-partitioning of Prochlorococcus populations in a stratified water column in the eastern North Atlantic Ocean. Appl Environ Microbiol 65:2585–2591
Acknowledgements
This work was supported by projects BOS2000-0139 and REN2000-0332-P4 from the Ministerio de Ciencia y Tecnología to J.M.; and I.F. was supported by a FPI fellowship from the Generalitat de Catalunya. E.O.C. benefits from the Programa Ramón y Cajal of the Spanish Ministerio de Ciencia y Tecnología. I.F. is especially grateful to Carles Borrego for his helpful comments about sulfur and pigment data and to Mike Gates for revising part of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrera, I., Massana, R., Casamayor, E.O. et al. High-diversity biofilm for the oxidation of sulfide-containing effluents. Appl Microbiol Biotechnol 64, 726–734 (2004). https://doi.org/10.1007/s00253-004-1582-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1582-x