Abstract
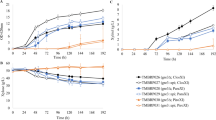
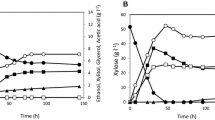
Two xylose-fermenting glucose-derepressed Saccharomyces cerevisiae strains were constructed in order to investigate the influence of carbon catabolite repression on xylose metabolism. S. cerevisiae CPB.CR2 (Δmig1, XYL1, XYL2, XKS1) and CPB.MBH2 (Δmig1, Δmig2, XYL1, XYL2, XKS1) were analysed for changes in xylose consumption rate and ethanol production rate during anaerobic batch and chemostat cultivations on a mixture of 20 g l−1 glucose and 50 g l−1 xylose, and their characteristics were compared to the parental strain S. cerevisiae TMB3001 (XYL1, XYL2, XKS1). Improvement of xylose utilisation was limited during batch cultivations for the constructed strains compared to the parental strain. However, a 25% and 12% increased xylose consumption rate during chemostat cultivation was achieved for CPB.CR2 and CPB.MBH2, respectively. Furthermore, during chemostat cultivations of CPB.CR2, where the cells are assumed to grow under non-repressive conditions as they sense almost no glucose, invertase activity was lower during growth on xylose and glucose than on glucose only. The 3-fold reduction in invertase activity could only be attributed to the presence of xylose, suggesting that xylose is a repressive sugar for S. cerevisiae.


Similar content being viewed by others
References
Christensen LH, Schulze U, Nielsen J, Villadsen J (1995) Acoustic gas analysis for fast and precise monitoring of bioreactors. Chem Eng Sci 50:2601–2610
Diderich JA, Schepper M, Van Hoek P, Luttik MA, van Dijken JP, Pronk JT, Klaassen P, Boelens HF, De Mattos MJ, van Dam K, Kruckeberg AL (1999) Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J Biol Chem 274:15350–15359
Dynesen J, Smits HP, Olsson L, Nielsen J (1998) Carbon catabolite repression of invertase during batch cultivations of Saccharomyces cerevisiae: the role of glucose, fructose, and mannose. Appl Microbiol Biotechnol 50:579–582
Eliasson A, Christensson C, Wahlbom CF, Hahn-Hägerdal B (2000) Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl Environ Microbiol 66:3381–3386
Gancedo JM (1992) Carbon catabolite repression in yeast. Eur J Biochem 206:297–313
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62:334–361
Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20:1425
Güldener U, Heck S, Fiedler T, Beinhauer J, Hegemann JH (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 24:2519–2524
Hamacher T, Becker J, Gárdonyi M, Hahn-Hägerdal B, Boles E (2002) Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilisation. Microbiology 148:2783–2788
Hernández-Montalvo V, Valle F, Bolivar F, Gosset G (2001) Characterization of sugar mixtures utilisation by an Escherichia coli mutant devoid of the phosphotransferase system. Appl Microbiol Biotechnol 57:186–191
Herwig C, Doerris C, Marison I, von Stockar U (2001) Quantitative analysis of the regulation scheme of invertase expression in Saccharomyces cerevisiae. Biotechnol Bioeng 76:247–258
Klein CJL, Olsson L, Nielsen J (1998) Glucose control in Saccharomyces cerevisiae: the role of MIG1 in metabolic functions. Microbiology 144:13–24
Klein CJL, Rasmussen JJ, Rønnow B, Olsson L, Nielsen J (1999) Investigation of the impact of MIG1 and MIG2 on the physiology of Saccharomyces cerevisiae. J Biotechnol 68:197–212
Kötter P, Ciriacy M (1993) Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 38:776–783
Kotyk A (1967) Properties of the sugar carrier in baker's yeast. Folia Microbiol 12:121–131
Kruckeberg AL, Bisson LF (1990) The HXT2 gene of Saccharomyces cerevisiae is required for high-affinity transport. Mol Cell Biol 10:5903–5913
Lundin M, Nehlin JO, Ronne H (1994). Importance of a flanking AT-rich region in target site recognition by the GC box-binding zinc finger protein MIG1. Mol Cell Biol 14:1979–1985
Lutfiyya LL, Johnston M (1996) Two zinc-finger-containing repressors are responsible for glucose repression of SUC2 expression. Mol Cell Biol 16:4790–4797
Nehlin JO, Ronne H (1990) Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J 9:2891–2898
Nichols NN, Dien BS, Bothast RJ (2001) Use of catabolite repression mutants for fermentation of sugars mixtures to ethanol. Appl Microbiol Biotechnol 56:120–125
Østergaard S, Olsson L, Johnston M, Nielsen J (2000) Increasing galactose consumption by Saccharomyces cerevisiae through metabolic engineering of the GAL gene regulatory network. Nat Biotechnol 18:1283–1286
Özcan S, Vallier LG, Flick JS, Carlson M, Johnston M (1997) Expression of SUC2 gene of Saccharomyces cerevisiae is induced by low level of glucose. Yeast 13:127–137
Reifenberger E, Boles E, Ciriacy M (1997) Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem 245:324–333
Richard P, Toivari MH, Penttilä M (1999) Evidence that the gene YLR070c of Saccharomyces cerevisiae encodes a xylitol dehydrogenase. FEBS Lett 457:135–138
Rizzi M, Erlemann P, Bui-Thanh N-A, Dellweg H (1988) Xylose fermentation by yeasts. 4. Purification and kinetic studies of xylose reductase from Pichia stipitis. Appl Microbiol Biotechnol 29:148–154
Roca C, Olsson L (2003) Increasing ethanol productivity during xylose fermentation by cell recycling of recombinant Saccharomyces cerevisiae. Appl Microbiol Biotechnol 60:560–563
Theodoris G, Fong NM, Coons DM, Bisson L (1994) High-copy suppression of glucose transport defects by HXT4 and regulatory elements in the promoters of the HXT genes in Saccharomyces cerevisiae. Genetics 137:957–966
Trumbly RJ (1992) Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol 6:15–21
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1990) Physiology of Saccharomyces cerevisiae in anaerobic glucose- limited chemostat cultures. J Gen Microbiol 136:395–403
Winde JH de, Grivell LA (1993) Global regulation of mitochondrial biogenesis in Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol 46:51–91
Acknowledgements
Professor Bärbel Hahn-Hägerdal, Department of Applied Microbiology, Lund University is sincerely thanked for kindly providing the plasmid carrying the XYL1, XYL2 and XKS1 genes and the S. cerevisiae strain TMB3001. We are also indebted to Juana M. Gancedo for communicating her unpublished results on the capacity of xylose to cause catabolite repression. The work on carbon catabolite repression at the Centre for Process Biotechnology at the Technical University of Denmark is supported under the European Commission Framework V, contract no. QLK3-CT-1999-00080.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roca, C., Haack, M.B. & Olsson, L. Engineering of carbon catabolite repression in recombinant xylose fermenting Saccharomyces cerevisiae . Appl Microbiol Biotechnol 63, 578–583 (2004). https://doi.org/10.1007/s00253-003-1408-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1408-2