Abstract
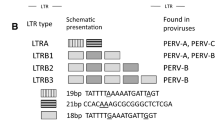
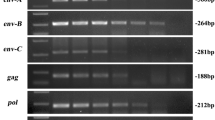
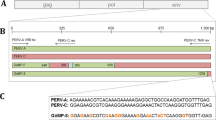
The pig (Sus scrofa) is a potential organ donor for man but porcine endogenous retroviruses (PERVs) represent an important concern for patients, and identification or engineering of PERV-free pigs suitable for xenotransplantation is a major undertaking. Consequently, studies of variability in pigs for the presence of PERVs at specific loci are a prerequisite. We identified genomic flanking sequences of two PERVs cloned in bacterial artificial chromosomes, a replication-competent PERV-A at locus 1q2.4 and a defective PERV-B at locus 7p1.1–2. PERV-A is embedded in the second repeat of a tandem of eight 190 bp repeats. A short duplicated 4 bp cellular motif, AGAC, was found at each flank of PERV-A and a degenerate 4 bp motif was found for PERV-B. At each locus, the PERV flanks matched expressed sequence tags available in public databases. Primer pairs were designed to amplify either genomic flanks or PERV-genomic junctions. Polymerase chain reaction screening was performed on pigs from 11 distinct Chinese breeds and from the European Large White breed. PERV-B at locus 7p1.1–2 was detected in all animals whereas the presence of PERV-A at locus 1q2.4 was variable. Our results suggest that a genetic selection can be designed to identify animals lacking a potentially active PERV at a specific locus and that Chinese and European pig breeds represent large biodiversity reservoirs to explore. Our results point also to the existence of PERVs that might be fixed in the pig genome, and that might not be eliminated by classical genetic selection.




Similar content being viewed by others
References
Akiyoshi DE, Denaro M, Zhu H, Greenstein JL, Banerjee P, Fishman JA (1998) Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol 72:4503–4507
Blusch JH, Seelmeir S, von der Helm K (2002) Molecular and enzymatic characterization of the porcine endogenous retrovirus protease. J Virol 76:7913–7917
Clémenceau B, Jegou D, Martignat L, Sai P (2002) PERV infection of mouse and human cells by SFP pig islets in nude mice. Diabetologia 45:914–923
Coffin JM (1996) Retroviridae; the viruses and their replication. In: Fields BN, Knipe DM, Howley PM (eds) Fields Virology, 3rd edn. Lippincott-Raven, Philadelphia, Md, pp 1767–1847
Czauderna F, Fisher N, Boller K, Kurth R, Tönjes RR (2000) Establishment and characterization of molecular clones of porcine endogenous retroviruses replicating on human cells. J Virol 74:4028–4038
Deng YM, Tuch BE, Rawlinson WD (2000a) Transmission of porcine endogenous retroviruses in severe combined immunodeficient mice xenotransplanted with fetal porcine pancreatic cells. Transplantation 70:1010–1016
Deng, YM, Lee JH, Moran C, Jin JH, Tuch BE, Rawlinson WD (2000b) Mapping dispersed repetitive loci using semi-specific PCR cloning and somatic cell hybrid mapping. Nucleic Acids Res 28:E103
Heneine W, Tibell A, Switzer WM, Sandstrom P, Vazquez Rosales G, Mathews A, Korsgren O, Chapman LE, Folks TM, Groth CG (1998) No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet 352:695–699
Herring C, Quinn G, Bower R, Parsons N, Logan NA, Brawley A, Elsome K, Whittam A, Fernandez-Suarez XM, Cunningham D, Onions D, Langford G, Scorbie L (2001) Mapping full-length porcine endogenous retroviruses in a Large White pig. J Virol 75:12252–12265
Hughes SH, Mutschler A, Bishop JM, Varmus HE (1981) A Rous sarcoma virus provirus is flanked by short direct repeats of a cellular DNA sequence present in only one copy prior to integration. Proc Natl Acad Sci U S A 78:4299–4303
Krach U, Fischer N, Czauderna F, Tönjes RR (2001) Comparison of replication-competent molecular clones of porcine endogenous retrovirus class A and class B derived from pig and human cells. J Virol 75:5465–5472
Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS (2002) Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089–1092
Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA (1997) Two sets of human-tropic pig retrovirus. Nature 389:681–682
Lee JH, Webb GC, Allen RDM, Moran C (2002) Characterizing and mapping porcine endogenous retroviruses in Westran pigs. J Virol 76:5548–5556
Martin U, Steinhoff G, Kiessig V, Chikobava M, Anssar M, Morschheuser T, Lapin B, Haverich A (1998) Porcine endogenous retrovirus (PERV) was not transmitted from transplanted porcine endothelial cells to baboons in vivo. Transplant Int 11:247–251
Martin U, Steinhoff G, Kiessig V, Chikobava M, Anssar M, Morschheuser T, Lapin B, Haverich A (1999) Porcine endogenous retrovirus is transmitted neither in vivo nor in vitro from porcine endothelial cells to baboons. Transplant Proc 31:913–914
Niebert M, Rogel-Gaillard C, Chardon P, Tönjes RR (2002) Characterization of chromosomally assigned replication-competent gamma porcine endogenous retroviruses derived from a Large White pig and expression in human cells. J Virol 76:2714–2720
Nikbakht KN, Boone LR, Glover PL, Myer FE, Yang WK (1987) Characterization of a molecular clone of RFM/Un mouse chromosomal DNA that contains a full-length endogenous murine leukaemia virus-related proviral genome. J Gen Virol 68:683–693
Oldmixon BA, Wood JC, Ericsson TA, Wilson CA, White-Scharf ME, Andersson G, Greenstein JL, Schuurman HJ, Patience C (2002) Porcine endogenous retrovirus transmission characteristics of an inbred herd of miniature swine. J Virol 76:3045–3048
Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, the XEN 111 Study Group, Otto E (1999) Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science 285:1236–1241
Patience C, Takeuchi Y, Weiss RA (1997) Infection of human cells by an endogenous retrovirus of pigs. Nat Med 3:282–286
Patience C, Patton GS, Takeuchi Y, Weiss RA, McClure MO, Rydberg L, Breimer ME (1998) No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet 352:699–701
Patience C, Switzer WM, Takeuchi Y, Griffiths DJ, Goward ME, Heneine W, Stoye JP, Weiss RA (2001) Multiple groups of novel retroviral genomes in pigs and related species. J Virol 75:2771–2775
Qari SH, Magre S, Garcia-Lerma JG, Hussain AI, Takeuchi Y, Patience C, Weiss RA, Heneine W (2001) Susceptibility of the porcine endogenous retrovirus to reverse transcriptase and protease inhibitors. J Virol 75:1048–1053
Rogel-Gaillard C, Bourgeaux N, Billault A, Vaiman M, Chardon P (1999) Construction of a swine BAC library: application to the characterization and mapping of porcine type C endoviral elements. Cytogenet Cell Genet 85:205–211
Rogel-Gaillard C, Hayes H, Bourgeaux N, Chardon P (2001) Assignment of two new loci for gamma 1 porcine endogenous retroviruses (γ1 PERV) to pig chromosome bands 2q21 and 11q12 by in situ hybridization. Cytogenet Cell Genet 95:112–113
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Scherdin U, Rhodes K, Breindl M (1990) Transcriptionally active genome regions are preferred targets for retroviral integration. J Virol 64:907–912
Shih CC, Stoye JP, Coffin JM (1988) Highly preferred targets for retrovirus integration. Cell 53:531–537
Takeuchi Y, Patience C, Magre S, Weiss RA, Banerjee PT, Le Tissier P, Stoye JP (1998) Host range and interference studies of three classes of pig endogenous retrovirus. J Virol 72:9986–9991
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tristem M, Kabat P, Lieberman L, Linde S, Karpas A, Hill F (1996) Characterization of a novel murine leukemia virus-related subgroup within mammals. J Virol 70:8241–8246
van der Laan LJW, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, Hering BJ, Long Z, Otto E, Torbett BE, Salomon DR (2000) Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature 407:90–94
Wilson CA, Wong S, Muller J, Davidson CE, Rose TM, Burd P (1998) Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol 72:3082–3087
Wilson CA, Wong S, VanBrocklin M, Federspiel MJ (2000) Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J Virol 74:49–56
Weiss RA (1998) Transgenic pigs and virus adaptation. Nature 391:327–328
Acknowledgements
This work was supported by the Department of Animal Genetics at INRA (France), the State Major Basic Research Development Program 973, project G2000016100 (China) and a cooperation program between France and China (PRA BT 99-05). The authors are grateful to M. Vaiman for critical reading of the manuscript and to H. Hayes and D. Lawrence for corrections. The experiments were performed in France and comply with current French laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Accession numbers: Nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank databases under Accession numbers AY160111–AY160114
Rights and permissions
About this article
Cite this article
Gorbovitskaia, M., Liu, Z., Bourgeaux, N. et al. Characterization of two porcine endogenous retrovirus integration loci and variability in pigs. Immunogenetics 55, 262–270 (2003). https://doi.org/10.1007/s00251-003-0579-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-003-0579-4