Abstract
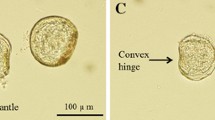
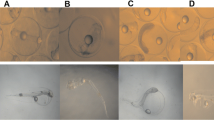
The tertiary branched alkyl-chain isomers of p-nonylphenol (NP) are perceived to have more estrogenic potency than its constituent secondary and primary straight alkyl-chain isomers. Investigations with single tertiary branched isomers of NP can therefore contribute toward the elucidation of the mechanisms of toxicity and estrogenicity of NP. A single tertiary branched alkyl-chain isomer (4(3′,6′-dimethyl-3′-heptyl)-phenol) was used in studies to determine its effects on embryonic growth and mortality in Lymnaea stagnalis L. Egg masses were exposed to the test compound for 20 days in a static waterborne-exposure regime with an average NP concentration of 105 μg/L and water temperature range of 18–20°C. Observations were made under a microscope and pictures were taken with a digital camera to determine the various developmental stages of growth, the duration of growth in each stage, embryo hatchability, and embryo mortality. The isomer was found to cause significant delay in all stages of growth and more significantly in the Morula and Veliger stages. An increase in embryo mortality, from the third day until the end of the experiment, was observed in exposed egg masses compared to controls. The hatching success of embryos was also significantly reduced by exposure, with 81% hatchability in exposed egg masses compared to 93% in the controls, after 18 days of continuous exposure. The encapsulating jelly strand that completely covers the rows of egg masses may have prevented the isomer residues from effectively penetrating into the embryos as shown by the observed low bioconcentration factors of the isomer in egg masses during exposure, resulting in unexpectedly lower observed estrogenic effects. However, this factor was not investigated. In vivo biotransformation of some of the residues of the isomer into catechol metabolites by the embryos during exposure could also result in the reduction of its estrogenic potential. To understand more fully the extent of toxicity and estrogenicity of this isomer, in vitro estrogenic assays are recommended. It would also be necessary to investigate its estrogenic effects on embryo development after in vivo maternal exposure.






Similar content being viewed by others
References
Arcaud-Hoy LD, Benson WH (1997) Fish reproduction: an ecologically relevant indicator of endocrine disruption. Environ Toxicol Chem 17:49–57
Arukwe A, Goksoyr A (1997) Xenobiotic and steroid biotransformation enzymes in Atlantic salmon (Salmo salar) liver treated with an estrogenic compound, 4-nonylphenol. Environ Toxicol Chem 16:2576–2583
Arukwe A, Thibaut R, Ingebrigtsen K, Celius T, Goksoyr A, Cravedi J-P (2000) In vivo and in vitro metabolism and organ distribution of nonylphenol in Atlantic salmon (Salmo salar). Aquat Toxicol 49:289–304
Atienzar FA, Billinghurst Z, Depledge MH (2002) 4-Nonylphenol and 17β-estradiol may induce common DNA effects in developing barnacle larvae. Environ Pollut 120:735–738
Bhatt BD, Prasad JV, Kalpana G, Ali S (1992) Separation and characterization of isomers of p-nonylphenols by capillary GC/MS-MS/GC-FTIR techniques. J Chromat Sci 30:203–210
Bluzart R, Seuge J (1981) Long-term effects of four detergents on fresh water pulmonates, Lymnaea stagnalis L.: Intoxication of the organisms from time of hatching. Environ Pollut 25:105–122
Brzozowski AM, Pike ACW, Dauter Z, Hubbard RE, Bonn T, Engstroem O, Oehman L, Green GL, Gustafsson JA, Carlquist M (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758
Canton JH, Slooff W (1977) The usefulness of Lymnaea stagnalis L. as a biological indicator in toxicological bio-assays (model substance γ-HCH). Water Res 11:117–121
Czech P, Weber K, Dietrich DR (2001) Effects of endocrine modulating substances on reproduction in Lymnaea stagnalis L. Aquat Toxicol 53:103–114
Ekelund R, Bergman A, Granmo A, Berggren M (1990) Bioaccumulation of 4-nonylphenol in marine animals: a Re-evaluation. Environ Pollut 64:107–120
Ferreira-Leach AMR, Hill EM (2001) Bioconcentration and distribution of 4-tert-octylphenol residues in tissues of the rainbow trout (Oncorhynchus mykiss). Mar Environ Res 51:75–89
Gagne F, Blaise C (1998) Estrogenic properties of municipal and industrial wastewaters evaluated with rapid and sensitive chemoluminescent in situ hybridisation assay (CISH) in rainbow trout hepatocytes. Aquat Toxicol 44:83–91
Gallagher EP, Gross TS, Sheely KM (2001) Decreased glutathione S-transferase expression and activity and altered sex steroids in Lake Apopka brown bullheads (Ameiurus nebulosus). Aquat Toxicol 55:223–237
Geraerts WPM, Joose J (1975) Control of vitellogenesis and of growth of female accessory organs by the Dorsal Body Hormone (DBH) in the Hermaphroditic freshwater snail Lymnaea stagnalis L. Gen Compar Endocrinol 27:450–464
Gray MA, Metcalf CD (1996) Induction of testis-ova in Japanese Medaka (Oryzias Latipes) exposed to p-nonylphenol. Environ Toxicol Chem 16:1082–1086
Harries JE, Sheahan DA, Jobling S, Matthiessen P, Neall P, Sumpter JP, Tyler T, Zaman N (1997) Estrogenic activity in five United Kingdom rivers detected by measurement of vitellogenesis in caged male trout. Environ Toxicol Chem 16:534–542
Helaleh MIH, Fujii S, Korenaga T (2001) Column silylation method for determining endocrine disruptors from environmental water samples by solid phase micro-extraction. Talanta 54:1039–1047
Hu J-Y, Aizawa T (2003) Quantitative structure-activity relationships for estrogen receptor binding affinity of phenolic chemicals. Water Res 37:1213–1222
Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP (1996) Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem 15:194–202
Jobling S, Sumpter JP (1993) Detergent components in sewage are weakly estrogenic to fish: An in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol 27:361–372
Koerner W, Spengler P, Bolz U, Schuller W, Hang V, Metzger JW (2001) Substances with estrogenic activity in effluents of sewage treatment plants in south western Germany: 2. Biological effects. Environ Toxicol Chem 20:2142–2151
Kvestak R, Ahel M (1994) Occurrence of toxic metabolites from non-ionic surfactants in the Krka river estuary. Ecotoxicol Environ Saf 28:25–34
Lalah JO, Lenoir D, Henkelmann B, Hertkorn N, Guenther K, Schramm K-W, Kettrup A (2001a) Synthesis of ring 14C-labelled 4(3′-,6′-dimethyl-3′-heptyl)-phenol. J Labelled Compd Radiopharm 44:459–463
Lalah JO, Lenoir D, Henkelmann B, Hertkorn N, Matuschek G, Schramm K-W, Guenther K, Kettrup A (2001b) Regioselective synthesis of a branched isomer of nonylphenol, 4(3′-, 6′-dimethyl-3′-heptyl)-phenol, and determination of its important environmental properties. Chem Eur J 7:4790–4795
Lalah JO, Schramm K-W, Behechti A, Henkelmann B, Lenoir D, Guenther K, Kettrup A (2003a) The dissipation, distribution and fate of a branched 14C-nonylphenol isomer in lake water/sediment systems. Environ Pollut 122:195–203
Lalah JO, Severin GF, Schramm K-W, Lenoir D, Henkelmann B, Behechti A, Guenther K, Kettru A (2003b) In vivo metabolism and organ distribution of a branched 14C-nonylphenol isomer in pond snails, Lymnaea stagnalis L. Aquat Toxicol 62:305–319
Lanzer R (1999) Toxicity of nonylphenol during embryo growth in Lymnaea stagnalis L. GSF Report. Institut für Ökologische Chemie, GSF-Forschungszentrum für Umwelt und Gesundheit, Neuherberg, Munich, Germany, 77 pp
Luchtel AW, Marti AW, Deyrup-Olsen I, Boer HH (1997) Gastropoda: Pulmonata. In: Harrison FW, Kohn AJ (eds) Microscopic anatomy of invertebrates, Mollusca, vol 6B. John Wiley, New York, pp 459–718
Madigou T, Le Goff P, Salbert G, Cravedi JP, Segner H, Pakdel F, Valotaire Y (2001) Effects of nonylphenol on estrogen-receptor conformation, transcriptional activity and sexual reversion in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 53:173–186
Meldahl AC, Nithipatikom K, Lech JJ (1996) Metabolism of several 14C-nonylphenol isomers by rainbow trout (Oncorhynchus mykiss): in vivo and in vitro microsomal metabolites. Xenobiotica 26:1167–1180
Mueller G, Kim UH (1978) Displacement of estradiol from estrogenic receptors by simple alkylphenols. Endocrinology 102:1429–1443
Muenzinger A (1987) Biomphalaria glabrata, a suitable organism for a biotest. Environ Technol Lett 8:141–148
Naylor GG, Mieure J, Adams WJ, Weeks JA, Castaldi FJ, Ogle LD, Romano RR (1992) Alkylphenol ethoxylates in the environment. J Am Oil Chemists 69:695–703
Pedersen SN, Christiansen LB, Pedersen KL, Korgaard B, Bjerregaard P (1999) In vivo estrogenic activity of branched and linear alkylphenols in rainbow trout (Oncorhynchus mykiss). Sci Total Environ 233:89–96
Routledge EJ, Sumpter JP (1997) Structural features of alkyphenolic chemicals associated with estrogenic activity. J Biol Chem 272:3280–3288
Schutz TW, Sinks GD (2002) Xenoestrogenic gene expression: structural features of active polycyclic aromatic hydrocarbons. Environ Toxicol Chem 21:783–786
Snyder SA, Keith TC, Naylor CG, Staples CA, Giesy JP (2001) Identification and quantification method for nonylphenol and lower oligomer nonylphenol ethoxylates in fish tissues. Environ Toxicol Chem 20:1870–1873
Soto AM, Justicia H, Wray JW, Sonnenschein C (1991) P-Nonylphenol: an estrogenic xenobiotic released from modified polystyrene. Environ Health Perspect 92:167–173
Spengler P, Koerner W, Metzger JW (2001) Substances with estrogenic activity in effluents of sewage treatment plants in Southwestern Germany. 1. Chemical analysis. Environ Toxicol Chem 20:2133–2141
Staples CA, Weeks J, Hall JF, Naylor CG (1998) Evaluation of aquatic toxicity and bioaccumulation of C8- and C9-alkylphenol ethoxylates. Environ Toxicol Chem 17:2470–2480
Thomas KV, Hurst MR, Matthiessen P, Waldock MJ (2001) Characterization of estrogenic compounds collected from the United Kingdom estuaries. Environ Toxicol Chem 20:2165–2170
Van Aerle R, Nolan M, Jobling S, Christiansen LB, Sumpter JP, Tylor CR (2001) Sexual disruption in a second species of wild cyprinid fish (the gudgeon, Gobio gobio) in United Kingdom freshwaters. Environ Toxicol Chem 20:2841–2847
Wheeler TF, Heim JR, LaTorre MR, Janes AB (1997) Mass spectral characterization of p-nonylphenol isomers using High Resolution Capillary GC-MS. J Chromat Sci 35:19–30
White R, Jobling S, Hoare SA, Sumpter JP, Paker MG (1994) Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 135:175–182
Yokota HF, Seki M, Maeda M, Oshima Y, Tadokoro H, Honjo T (2001) Life-cycle toxicity of 4-nonylphenol to Medaka (Oryzias Latipes). Environ Toxicol Chem 20:2552–2560
Acknowledgment
The authors would like to express their sincere gratitude to the Alexander von Humboldt Foundation of Germany for a Georg Forster research fellowship to Joseph O. Lalah. Sincere thanks to Sarah Ronkholz and Christine Harzer of Munich Technical University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lalah, J.O., Severin, G.F., Schramm, KW. et al. Effects of a Branched p-Nonylphenol Isomer (4(3′,6′-dimethyl-3′-heptyl)-phenol) on Embryogenesis in Lymnae stagnalis L.. Arch Environ Contam Toxicol 52, 104–112 (2007). https://doi.org/10.1007/s00244-004-0228-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0228-4