Abstract
Aim
To gather information on anticoagulant effects after the termination of long-term therapy with idraparinux.
Methods
The anticoagulant effects of idraparinux, a synthetic polymethylated analogue of its pentasaccharide, were analysed in 23 patients after termination of a 6- or 12-month therapy period for the prevention of recurrent venous thromboembolism (VTE). Plasma samples of patients initially randomized to 2.5 mg idraparinux (normal creatinine clearance) or 1.5 mg idraparinux (creatinine clearance <30 ml/min) were investigated in the van Gogh trials. At 3-month intervals for up to 15 months following the termination of the therapy, the factor Xa-specific S2222 chromogenic substrate (aXa) assay and Heptest were used to determine various pharmacokinetic parameters and prothrombin-induced clotting time (PiCT), activated partial thromboplastin time (aPTT) and prothrombin time (PT) were determined.
Results
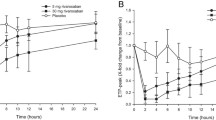
Based on the aXa assay and Heptest, the half lives (t1/2) were 60.2 days and 107.7 days (p < 0.0001), maximum drug concentrations (Cmax) were 0.30 and 0.39 μg/l (p = 0.0016), areas under the activity time curve (AUC) were 33.7 and 38.0 μg/l per day (p = 0.0002), plasma clearances were 0.09 and 0.06 ml/min (p < 0.0001), mean residence times (MRT) were 75.4 and 121.9 (p < 0.0001) and volumes of distribution (Vdiss) were 7.4 and 8.6 l (p = 0.1336), respectively. After 12 months of treatment (n = 18), the S2222 and Heptest results showed significantly higher Cmax and AUC, lower Vdiss and clearance and unchanged t1/2 and MRT values compared to 6 months of treatment (n = 5). The PiCT was prolonged for a period of 9 months. Coagulation times of aPTT and PT were not influenced. The results of these parameters did not differ between 12 and 6 months of treatment.
Conclusion
The data support reports on a non-ionic binding of idraparinux to antithrombin and other proteins. We suggest that these findings may explain some of the findings of the van Gogh Extension trial.



Similar content being viewed by others
References
Hirsh J, Raschke R (2004) Heparin and low-molecular-weight heparin: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126:188S–203S
Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE (2004) Antithrombotic therapy for venous thromboembolic disease: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126:401S–428S
Warkentin TE, Greinacher A (2004) Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126:311S–337S
Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P, MacKinnon B, Julian JA (1999) A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 340:901–907
Ansel Jl, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E (2004) The pharmacology and management of the vitamin K antagonists: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126:204S–233S
Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, MacKinsson B, Weitz JI, Crowther MA, Dolan S, Turpie AG, Geerts W, Solymoss S, van Nguyen P, Demers C, Kahn SR, Kassis J, Rodger M, Hambleton J, Gent M (2003) Extended low-intensity anticoagulation for thrombo-embolism investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 349:631–639
Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, Cushman M, Moll S, Kessler CM, Elliott CG, Paulson R, Wong T, Bauer KA, Schwartz BA, Miletich JP, Bounameaux H, Glynn RJ (2003) PREVENT Investigators. Long-term, long-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 348:1425–1434
Schulman S, Wahlander K, Lundstrom T, Clason SB, Eriksson H, THRIVE III Investigators (2003) Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 349:1713–1721
Weitz JI, Bates SM (2005) New anticoagulants. J Thromb Haemost 3:1843–1853
Herbert JM, Herault JP, Bernat A et al (1988) Biochemical and pharmacological properties of SANORG 34006, a potent and long-acting synthetic pentasaccharide. Blood 91:4197–4205
de Kort M, Buijsman RC, van Boeckel CA (2005) Synthetic heparin derivatives as new anticoagulant drugs. Drug Discov Today 10:769–779
Ma Q, Fareed J (2004) Idraparinux sodium. Sanofi-Aventis. IDrugs 7:1028–1034
Persist Investigators (2004) A novel long-acting synthetic factor Xa inhibitor (SanOrg340006) to replace warfarin for secondary prevention in deep vein thrombosis: a Phase II evaluation. J Thromb Haemost 2:47–53
The van Gogh Investigators (2007) Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med 357:1094–1104
The van Gogh Investigators (2007) Extended prophylaxis of venous thromboembolism with idraparinux. N Engl J Med 357:1105–1112
Harenberg J, Giese C, Dempfle CE, Stehle G, Heene DL (1988) Monitoring of heparin and low molecular weight heparin with capillary and venous whole blood. Thromb Haemost 60:377–381
Harenberg J, Giese C, Hagedorn A, Traeger I, Fenyvesi T (2007) Determination of antithrombin-dependent factor Xa inhibitors by prothrombin-induced clotting time. Semin Thromb Hemost 33:503–507
Oberwittler H, Hirschfeld-Warneken A, Wesch R, Willerich H, Teichert L, Lehr KH, Ding R, Haefeli WE, Mikus G (2007) Significant pharmacokinetic and pharmacodynamic interaction of warfarin with the NO-independent sGC activator HMR1766. J Clin Pharmacol 47:70–77
Van Amsterdam RGM, Burggraaf J, Borm GF, Schoemaker HC, de Greef HJMM, Faaij RA, Nelissen JMDT (2004) A phase I multiple dose study to investigate the safety, tolerance and pharmacokinetics of 10 mg SC SanOrg34006 in male and female volunteers with a history of VTE, recently discharged from oral anticoagulant treatment. Clinical Trial Report on Protocol 64703. R&DRR NL0028967. Organon, Oss
Fairclough A (2002) The in vitro binding of [35-S]-SanOrg3400S (SR34006A) in rat, monkey and human (normal and AT III depleted) plasma. Research report 767.5.017. Sanofi-Synthelabo, France
Hjelm R, Schedin-Weiss S (2007) High affinity interaction between a synthetic, highly negatively charged pentasaccharide and R- or â-antithrombin is predominantly due to nonionic interactions. Biochemistry 46:3378–3384
van Boeckel CAA, Petitou M (1993) The unique antithrombin III binding domain of heparin: a lead to newsynthetic antithrombotics. Angew Chem Int Ed Engl 32:1671–1818
Jeske W, Fareed J (1999) In vitro studies on the biochemistry and pharmacology of low molecular weight heparins. Semin Thromb Hemost 25 [Suppl 3]:27–33
Fenyvesi T, Jörg I, Harenberg J (2002) Effect of phenprocoumon on monitoring of lepirudin, argatroban, melagatran and unfractionated heparin with the PiCT method. Pathophysiol Haemost Thromb 32:174–179
Thomas DP, Merton RE, Lewis WE, Barrowcliffe TW (1981) Studies in man and experimental animals of a low molecular weight heparin fraction. Thromb Haemost 45:214–218
Harenberg J, Gnasso A, de Vries JX, Zimmermann R, Augustin J (1985) Anticoagulant and lipolytic effects of a low molecular weight heparin fraction. Thromb Res 39:683–692
Ma Q, Tobu M, Schultz C, Jeske W, Hoppensteadt D, Walenga J, Cornelli U, Lee J, Linhardt R, Hanin I, Fareed J (2007) Molecular weight dependent tissue factor pathway inhibitor release by heparin and heparin oligosaccharides. Thromb Res 119:653–661
Walenga JM, Jeske WP, Samama MM, Frapaise FX, Bick RL, Fareed J (2002) Fondaparinux: a synthetic heparin pentasaccharide as a new antithrombotic agent. Expert Opin Investig Drugs 11:397–407
Savi P, Herault JP, Duchaussoy P, Millet L, Petitou M, Schaeffer P, Bono F, Herbert JM (2007) Reversible biotinylated oligosaccharides, a new approch for a better management of the anticoagulant therapy (abstract). J Thromb Haemost [Suppl] P-W-645
Acknowledgment
The authors would like to thank Dr. H. Bratsch for his collaboration and Mrs. Ch. Giese, Mrs. A. Hagedorn and Mrs. I. Traeger for their excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harenberg, J., Jörg, I., Vukojevic, Y. et al. Anticoagulant effects of Idraparinux after termination of therapy for prevention of recurrent venous thromboembolism: observations from the van Gogh trials. Eur J Clin Pharmacol 64, 555–563 (2008). https://doi.org/10.1007/s00228-008-0463-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0463-0