Abstract
Copper plays an important role in numerous biological processes across all living systems predominantly because of its versatile redox behavior. Cellular copper homeostasis is tightly regulated and disturbances lead to severe disorders such as Wilson disease and Menkes disease. Age-related changes of copper metabolism have been implicated in other neurodegenerative disorders such as Alzheimer disease. The role of copper in these diseases has been a topic of mostly bioinorganic research efforts for more than a decade, metal–protein interactions have been characterized, and cellular copper pathways have been described. Despite these efforts, crucial aspects of how copper is associated with Alzheimer disease, for example, are still only poorly understood. To take metal-related disease research to the next level, emerging multidimensional imaging techniques are now revealing the copper metallome as the basis to better understand disease mechanisms. This review describes how recent advances in X-ray fluorescence microscopy and fluorescent copper probes have started to contribute to this field, specifically in Wilson disease and Alzheimer disease. It furthermore provides an overview of current developments and future applications in X-ray microscopic methods.

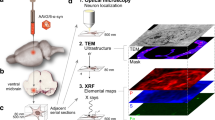
3 mm × 3 mm P, Fe, and Cu elemental maps of a lateral ventricle from a mouse brain. An H & E image is shown for comparison. The images are displayed as red temperature maps where lighter color indicates higher elemental concentration. The image emphasizes the power of XFM: the copper distribution around the lateral ventricle is extremely heterogenous with local copper concentrations exceeding 25 mM while the average is approximately 100 μM.




Similar content being viewed by others
Notes
One exciting development with regard to X-ray sources is the X-ray free-electron laser. X-ray free-electron lasers produce X-ray pulses with peak intensities that are approximately 109 times more intense than synchrotron radiation [28]. The main application of X-ray free-electron lasers is the determination of protein crystal structures from microcrystals or nanocrystals [29, 30]. In such an application (at least for now) X-ray free-electron lasers are used to collect X-ray diffraction patterns.
Abbreviations
- Aβ:
-
Amyloid β
- FTIRM:
-
Fourier transform infrared microspectroscopy
- LA-ICPMS:
-
Laser ablation inductively coupled mass spectrometry
- PIXE:
-
Proton-induced X-ray emission
- SIMS:
-
Secondary ion mass spectrometry
- XANES:
-
X-ray absorption near-edge spectroscopy
- XFM:
-
X-ray fluorescence microscopy
- XRM:
-
X-ray microscopy
References
Bourassa MW, Miller LM (2012) Metal imaging in neurodegenerative diseases. Metallomics
Glasauer S, Langley S, Boyanov M, Lai B, Kemner K, Beveridge TJ (2007) Mixed-valence cytoplasmic iron granules are linked to anaerobic respiration. Appl Environ Microbiol 73(3):993–996. doi:10.1128/AEM.01492-06
Kemner KM, Kelly SD, Lai B, Maser J, O'Loughlin EJ, Sholto-Douglas D, Cai Z, Schneegurt MA, Kulpa CF Jr, Nealson KH (2004) Elemental and redox analysis of single bacterial cells by x-ray microbeam analysis. Science 306(5696):686–687
Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ (2005) Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron x-ray fluorescence microscopy. Proc Natl Acad Sci USA 102(32):11179–11184. doi:10.1073/pnas.0406547102
Ortega R, Deves G, Carmona A (2009) Bio-metals imaging and speciation in cells using proton and synchrotron radiation X-ray microspectroscopy. J R Soc Interface 6(Suppl 5):S649–S658. doi:10.1098/rsif.2009.0166.focus
Fahrni CJ (2007) Biological applications of X-ray fluorescence microscopy: exploring the subcellular topography and speciation of transition metals. Curr Opin Chem Biol 11(2):121–127. doi:10.1016/j.cbpa.2007.02.039
Paunesku T, Vogt S, Irving TC, Lai B, Barrea RA, Maser J, Woloschak GE (2009) Biological applications of X-ray microprobes. Int J Radiat Biol 85(8):710–713. doi:10.1080/09553000903009514
Lobinski R, Moulin C, Ortega R (2006) Imaging and speciation of trace elements in biological environment. Biochimie 88(11):1591–1604. doi:10.1016/j.biochi.2006.10.003
Ralle M, Lutsenko S (2009) Quantitative imaging of metals in tissues. Biometals 22(1):197–205. doi:10.1007/s10534-008-9200-5
Jensen MP, Aryal BP, Gorman-Lewis D, Paunesku T, Lai B, Vogt S, Woloschak GE (2012) Submicron hard X-ray fluorescence imaging of synthetic elements. Anal Chim Acta 722:21–28. doi:10.1016/j.aca.2012.01.064
Paunesku T, Vogt S, Maser J, Lai B, Woloschak G (2006) X-ray fluorescence microprobe imaging in biology and medicine. J Cell Biochem 99(6):1489–1502. doi:10.1002/jcb.21047
Bohic S, Cotte M, Salome M, Fayard B, Kuehbacher M, Cloetens P, Martinez-Criado G, Tucoulou R, Susini J (2012) Biomedical applications of the ESRF synchrotron-based microspectroscopy platform. J Struct Biol 177(2):248–258. doi:10.1016/j.jsb.2011.12.006
Chen S, Flachenecker C, Lai B, Paunesku T, Roehrig C, Vonosinski J, Bolbat M, Maser J, Shu D, Finney L, Gleber S, Jin Q, Brister K, Jacobsen C, Vogt S, Woloschak G (2012) 2D/3D trace elemental mapping of frozen-hydrated biomaterials using the bionanoprobe. Paper presented at Microscopy and microanalysis 2012, Phoenix, 29 July to 2 August 2012
Matsuyama S, Mimura H, Yumoto H, Sano Y, Yamamura K, Yabashi M, Nishino Y, Tamasaku K, Ishikawa T, Yamauchi K (2006) Development of scanning x-ray fluorescence microscope with spatial resolution of 30 nm using Kirkpatrick-Baez mirror optics. Rev Sci Instrum 77(10):doi:10.1063/1.2358699
Ryan C, Siddons D, Moorhead G, Kirkham R, Dunn P, Dragone A, De Geronimo G (2007) Large detector array and real-time processing and elemental image projection of X-ray and proton microprobe fluorescence data. Nucl Instrum Meth Phys Res Sect B Beam Interact Mater Atoms 260:1–7
Chen KG, Valencia JC, Lai B, Zhang G, Paterson JK, Rouzaud F, Berens W, Wincovitch SM, Garfield SH, Leapman RD, Hearing VJ, Gottesman MM (2006) Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci USA 103(26):9903–9907. doi:10.1073/pnas.0600213103
Finney L, Mandava S, Ursos L, Zhang W, Rodi D, Vogt S, Legnini D, Maser J, Ikpatt F, Olopade OI, Glesne D (2007) X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc Natl Acad Sci U S A 104(7):2247–2252. doi:10.1073/pnas.0607238104
Howells MR, Beetz T, Chapman HN, Cui C, Holton JM, Jacobsen CJ, Kirz J, Lima E, Marchesini S, Miao H, Sayre D, Shapiro DA, Spence JCH, Starodub D (2009) An assessment of the resolution limitation due to radiation-damage in X-ray diffraction microscopy. J Electron Spectrosc Relat Phenom 170(1–3):4–12
Schrag M, Dickson A, Jiffry A, Kirsch D, Vinters HV, Kirsch W (2010) The effect of formalin fixation on the levels of brain transition metals in archived samples. Biometals 23(6):1123–1127. doi:10.1007/s10534-010-9359-4
James SA, Myers DE, de Jonge MD, Vogt S, Ryan CG, Sexton BA, Hoobin P, Paterson D, Howard DL, Mayo SC, Altissimo M, Moorhead GF, Wilkins SW (2011) Quantitative comparison of preparation methodologies for X-ray fluorescence microscopy of brain tissue. Anal Bioanal Chem 401(3):853–864. doi:10.1007/s00216-011-4978-3
Matsuyama S, Shimura M, Fujii M, Maeshima K, Yumoto H, Mimura H, Sano Y, Yabashi M, Nishino Y, Tamasaku K, Ishizaka Y, Ishikawa T, Yamauchi K (2010) Elemental mapping of frozen-hydrated cells with cryo-scanning X-ray fluorescence microscopy. X-Ray Spectrom 39(4):260–266. doi:10.1002/xrs.1256
Lai B, Chen S, Bolbat M, Jin Q, Finney L, Brister K, Jacobsen C, Vogt S (2012) A flexible cryojet-cooled X-ray fluorescence microprobe: initial results. Paper presented at Microscopy and microanalysis 2012, Phoenix, 29 July to 2 August 2012
de Jonge MD, Vogt S (2010) Hard X-ray fluorescence tomography–an emerging tool for structural visualization. Curr Opin Struct Biol 20(5):606–614. doi:10.1016/j.sbi.2010.09.002
de Jonge MD, Holzner C, Baines SB, Twining BS, Ignatyev K, Diaz J, Howard DL, Legnini D, Miceli A, McNulty I, Jacobsen CJ, Vogt S (2010) Quantitative 3D elemental microtomography of Cyclotella meneghiniana at 400-nm resolution. Proc Natl Acad Sci USA 107(36):15676–15680. doi:10.1073/pnas.1001469107
De Samber B, Silversmit G, De Schamphelaere K, Evens R, Schoonjans T, Vekemans B, Janssen C, Masschaele B, VanHoorebeke LS, Szalóki I et al (2010) Element-to-tissue correlation in biological samples determined by three-dimensional X-ray imaging methods. J Anal Atom Spectrom 25:544–553
Kim SA, Punshon T, Lanzirotti A, Li L, Alonso JM, Ecker JR, Kaplan J, Guerinot ML (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314(5803):1295–1298. doi:10.1126/science.1132563
McEwen BF, Downing KH, Glaeser RM (1995) The relevance of dose-fractionation in tomography of radiation-sensitive specimens. Ultramicroscopy 60(3):357–373
Schlichting I, Miao J (2012) Emerging opportunities in structural biology with X-ray free-electron lasers. Curr Opin Struct Biol. doi:10.1016/j.sbi.2012.07.015
Boutet S, Lomb L, Williams GJ, Barends TR, Aquila A, Doak RB, Weierstall U, DePonte DP, Steinbrener J, Shoeman RL, Messerschmidt M, Barty A, White TA, Kassemeyer S, Kirian RA, Seibert MM, Montanez PA, Kenney C, Herbst R, Hart P, Pines J, Haller G, Gruner SM, Philipp HT, Tate MW, Hromalik M, Koerner LJ, van Bakel N, Morse J, Ghonsalves W, Arnlund D, Bogan MJ, Caleman C, Fromme R, Hampton CY, Hunter MS, Johansson LC, Katona G, Kupitz C, Liang M, Martin AV, Nass K, Redecke L, Stellato F, Timneanu N, Wang D, Zatsepin NA, Schafer D, Defever J, Neutze R, Fromme P, Spence JC, Chapman HN, Schlichting I (2012) High-resolution protein structure determination by serial femtosecond crystallography. Science 337(6092):362–364. doi:10.1126/science.1217737
Seibert MM, Ekeberg T, Maia FR, Svenda M, Andreasson J, Jonsson O, Odic D, Iwan B, Rocker A, Westphal D, Hantke M, DePonte DP, Barty A, Schulz J, Gumprecht L, Coppola N, Aquila A, Liang M, White TA, Martin A, Caleman C, Stern S, Abergel C, Seltzer V, Claverie JM, Bostedt C, Bozek JD, Boutet S, Miahnahri AA, Messerschmidt M, Krzywinski J, Williams G, Hodgson KO, Bogan MJ, Hampton CY, Sierra RG, Starodub D, Andersson I, Bajt S, Barthelmess M, Spence JC, Fromme P, Weierstall U, Kirian R, Hunter M, Doak RB, Marchesini S, Hau-Riege SP, Frank M, Shoeman RL, Lomb L, Epp SW, Hartmann R, Rolles D, Rudenko A, Schmidt C, Foucar L, Kimmel N, Holl P, Rudek B, Erk B, Homke A, Reich C, Pietschner D, Weidenspointner G, Struder L, Hauser G, Gorke H, Ullrich J, Schlichting I, Herrmann S, Schaller G, Schopper F, Soltau H, Kuhnel KU, Andritschke R, Schroter CD, Krasniqi F, Bott M, Schorb S, Rupp D, Adolph M, Gorkhover T, Hirsemann H, Potdevin G, Graafsma H, Nilsson B, Chapman HN, Hajdu J (2011) Single mimivirus particles intercepted and imaged with an X-ray laser. Nature 470(7332):78–81. doi:10.1038/nature09748
Vogt S (2003) Maps: a set of software tools for analysis and visualization of 3D X-ray fluorescent datasets. J Phys IV 104:635–638
Solé VA, Papillon E, Cotte M, Walter P, Susini J (2007) A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta B 62:63–68. doi:10.1016/j.sab.2006.12.002
de Jonge MD, Hornberger B, Holzner C, Legnini D, Paterson D, McNulty I, Jacobsen C, Vogt S (2008) Quantitative phase imaging with a scanning transmission x-ray microscope. Phys Rev Lett 100(16):163902
Hornberger B, de Jonge MD, Feser M, Holl P, Holzner C, Jacobsen C, Legnini D, Paterson D, Rehak P, Struder L, Vogt S (2008) Differential phase contrast with a segmented detector in a scanning X-ray microprobe. J Synchrotron Radiat 15(4):355–362. doi:10.1107/S0909049508008509
Holzner C, Feser M, Vogt S, Hornberger B, Baines SB, Jacobsen C (2010) Zernike phase contrast in scanning microscopy with X-rays. Nat Phys 6(11):883–887
Leskovjan AC, Kretlow A, Lanzirotti A, Barrea R, Vogt S, Miller LM (2011) Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer's disease. Neuroimage 55(1):32–38. doi:10.1016/j.neuroimage.2010.11.073
Leskovjan AC, Kretlow A, Miller LM (2010) Fourier transform infrared imaging showing reduced unsaturated lipid content in the hippocampus of a mouse model of Alzheimer's disease. Anal Chem 82(7):2711–2716. doi:10.1021/ac1002728
Leskovjan AC, Lanzirotti A, Miller LM (2009) Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer's disease. Neuroimage 47(4):1215–1220. doi:10.1016/j.neuroimage.2009.05.063
Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J (2006) Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer's disease. J Struct Biol 155(1):30–37. doi:10.1016/j.jsb.2005.09.004
Miller LM, Wang Q, Smith RJ, Zhong H, Elliott D, Warren J (2007) A new sample substrate for imaging and correlating organic and trace metal composition in biological cells and tissues. Anal Bioanal Chem 387(5):1705–1715. doi:10.1007/s00216-006-0879-2
McRae R, Lai B, Vogt S, Fahrni CJ (2006) Correlative microXRF and optical immunofluorescence microscopy of adherent cells labeled with ultrasmall gold particles. J Struct Biol 155:22–29
Takizawa T, Suzuki K, Robinson JM (1998) Correlative microscopy using FluoroNanogold on ultrathin cryosections. Proof of principle. J Histochem Cytochem 46(10):1097–1102
Takizawa T, Robinson JM (2000) FluoroNanogold is a bifunctional immunoprobe for correlative fluorescence and electron microscopy. J Histochem Cytochem 48(4):481–486
Endres PJ, Macrenaris KW, Vogt S, Allen MJ, Meade TJ (2006) Quantitative imaging of cell-permeable magnetic resonance contrast agents using x-ray fluorescence. Mol Imaging 5(4):485–497
Endres PJ, MacRenaris KW, Vogt S, Meade TJ (2008) Cell-permeable MR contrast agents with increased intracellular retention. Bioconjug Chem 19(10):2049–2059. doi:10.1021/bc8002919
Endres PJ, Paunesku T, Vogt S, Meade TJ, Woloschak GE (2007) DNA-TiO2 nanoconjugates labeled with magnetic resonance contrast agents. J Am Chem Soc 129(51):15760–15761. doi:10.1021/ja0772389
Paunesku T, Ke T, Dharmakumar R, Mascheri N, Wu A, Lai B, Vogt S, Maser J, Thurn K, Szolc-Kowalska B, Larson A, Bergan RC, Omary R, Li D, Lu ZR, Woloschak GE (2008) Gadolinium-conjugated TiO2-DNA oligonucleotide nanoconjugates show prolonged intracellular retention period and T1-weighted contrast enhancement in magnetic resonance images. Nanomedicine 4(3):201–207. doi:10.1016/j.nano.2008.04.004
Paunesku T, Vogt S, Lai B, Maser J, Stojicevic N, Thurn KT, Osipo C, Liu H, Legnini D, Wang Z, Lee C, Woloschak GE (2007) Intracellular distribution of TiO2-DNA oligonucleotide nanoconjugates directed to nucleolus and mitochondria indicates sequence specificity. Nano Lett 7(3):596–601. doi:10.1021/nl0624723
Corezzi S, Urbanelli L, Cloetens P, Emiliani C, Helfen L, Bohic S, Elisei F, Fioretto D (2009) Synchrotron-based X-ray fluorescence imaging of human cells labeled with CdSe quantum dots. Anal Biochem 388(1):33–39. doi:10.1016/j.ab.2009.01.044
Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S (2010) Highly multiparametric analysis by mass cytometry. J Immunol Methods 361(1–2):1–20. doi:10.1016/j.jim.2010.07.002
Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ (2011) Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc Natl Acad Sci USA 108(15):5980–5985. doi:10.1073/pnas.1009932108
Domaille DW, Zeng L, Chang CJ (2010) Visualizing ascorbate-triggered release of labile copper within living cells using a ratiometric fluorescent sensor. J Am Chem Soc 132(4):1194–1195. doi:10.1021/ja907778b
Miller EW, Zeng L, Domaille DW, Chang CJ (2006) Preparation and use of Coppersensor-1, a synthetic fluorophore for live-cell copper imaging. Nat Protoc 1(2):824–827. doi:10.1038/nprot.2006.140
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 128(1):10–11. doi:10.1021/ja055064u
Taki M, Iyoshi S, Ojida A, Hamachi I, Yamamoto Y (2010) Development of highly sensitive fluorescent probes for detection of intracellular copper(I) in living systems. J Am Chem Soc 132(17):5938–5939. doi:10.1021/ja100714p
Hirayama T, Van de Bittner GC, Gray LW, Lutsenko S, Chang CJ (2012) Near-infrared fluorescent sensor for in vivo copper imaging in a murine Wilson disease model. Proc Natl Acad Sci USA 109(7):2228–2233. doi:10.1073/pnas.1113729109
Rurack K, Resch-Genger U (2002) Rigidization, preorientation and electronic decoupling–the 'magic triangle' for the design of highly efficient fluorescent sensors and switches. Chem Soc Rev 31(2):116–127
Chaudhry AF, Verma M, Morgan MT, Henary MM, Siegel N, Hales JM, Perry JW, Fahrni CJ (2010) Kinetically controlled photoinduced electron transfer switching in Cu(I)-responsive fluorescent probes. J Am Chem Soc 132(2):737–747. doi:10.1021/ja908326z
Verma M, Chaudhry AF, Morgan MT, Fahrni CJ (2010) Electronically tuned 1,3,5-triarylpyrazolines as Cu(I)-selective fluorescent probes. Org Biomol Chem 8(2):363–370. doi:10.1039/b918311f
Rubino JT, Franz KJ (2012) Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J Inorg Biochem 107(1):129–143. doi:10.1016/j.jinorgbio.2011.11.024
Bremner I (1998) Manifestations of copper excess. Am J Clin Nutr 67(5 Suppl):1069S–1073S
O'Halloran TV, Culotta VC (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275(33):25057–25060. doi:10.1074/jbc.R000006200
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284(5415):805–808
Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM et al (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5(4):344–350
Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D et al (1993) Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet 3(1):20–25
Issue T (2011) Metals in neurodegenerative disease. Metallomics 3(3):217–304
Banci L, Bertini I, Cantini F, Ciofi-Baffoni S (2010) Cellular copper distribution: a mechanistic systems biology approach. Cell Mol Life Sci 67(15):2563–2589. doi:10.1007/s00018-010-0330-x
Kim BE, Nevitt T, Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4(3):176–185. doi:10.1038/nchembio.72
Wang Y, Hodgkinson V, Zhu S, Weisman GA, Petris MJ (2011) Advances in the understanding of mammalian copper transporters. Adv Nutr 2(2):129–137. doi:10.3945/an.110.000273000273
Collins JF, Prohaska JR, Knutson MD (2010) Metabolic crossroads of iron and copper. Nutr Rev 68(3):133–147. doi:10.1111/j.1753-4887.2010.00271.x
Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5(4):327–337
Schushan M, Bhattacharjee A, Ben-Tal N, Lutsenko S (2012) A structural model of the copper ATPase ATP7B to facilitate analysis of Wilson disease-causing mutations and studies of the transport mechanism. Metallomics. doi:10.1039/c2mt20025b
Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY (2007) Function and regulation of human copper-transporting ATPases. Physiol Rev 87(3):1011–1046
Guo Y, Nyasae L, Braiterman LT, Hubbard AL (2005) NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol 289(5):G904–G916. doi:10.1152/ajpgi.00262.2005
Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF (2006) ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology 130(2):493–506. doi:10.1053/j.gastro.2005.10.054
Scott LD (1978) Copper toxicity in primary biliary cirrhosis. Gastroenterology 74(2 Pt 1):333–334
Brewer GJ (1998) Wilson disease and canine copper toxicosis. Am J Clin Nutr 67(5 Suppl):1087S–1090S
Brewer GJ, Yuzbasiyan-Gurkan V (1992) Wilson disease. Medicine (Baltimore) 71(3):139–164
Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC (1999) Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet 8(9):1665–1671
Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S (2006) Consequences of copper accumulation in the livers of the atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol 168(2):423–434
Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, Olson NE, Teupser D, Lutsenko S (2007) High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 282(11):8343–8355
Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP (1993) Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet 3(1):14–19
Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A (2007) Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am J Physiol Gastrointest Liver Physiol 292(4):G1181–G1194. doi:10.1152/ajpgi.00472.2006
Goldfischer S, Sternlieb I (1968) Changes in the distribution of hepatic copper in relation to the progression of Wilson's disease (hepatolenticular degeneration). Am J Pathol 53(6):883–901
Sternlieb I (1972) Evolution of the hepatic lesion in Wilson's disease (hepatolenticular degeneration). Prog Liver Dis 4:511–525
Goldfischer S (1967) Demonstration of copper and acid phosphatase activity in hepatocyte lysosomes in experimental copper toxicity. Nature 215(5096):74–75
Haywood S, Loughran M, Batt RM (1985) Copper toxicosis and tolerance in the rat. III. Intracellular localization of copper in the liver and kidney. Exp Mol Pathol 43(2):209–219
Scheinberg IH, Sternlieb I (1984) Wilson's disease. Major problems in internal medicine, vol XXIII. Saunders, Philadelphia
Ralle M, Huster D, Vogt S, Schirrmeister W, Burkhead JL, Capps TR, Gray L, Lai B, Maryon E, Lutsenko S (2010) Wilson's disease at a single cell level:intracellular copper trafficking activates compartment-specific responses in hepatocytes. J Biol Chem. doi:10.1074/jbc.M110.114447
Peng F, Lutsenko S, Sun X, Muzik O (2012) Positron emission tomography of copper metabolism in the Atp7b(−)/(−) knock-out mouse model of Wilson's disease. Mol Imaging Biol 14(1):70–78. doi:10.1007/s11307-011-0476-4
Hamza I, Schaefer M, Klomp LW, Gitlin JD (1999) Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci USA 96(23):13363–13368
Hamza I, Prohaska J, Gitlin JD (2003) Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci USA 100(3):1215–1220. doi:10.1073/pnas.0336230100
Walker JM, Tsivkovskii R, Lutsenko S (2002) Metallochaperone Atox1 transfers copper to the NH2-terminal domain of the Wilson's disease protein and regulates its catalytic activity. J Biol Chem 277(31):27953–27959. doi:10.1074/jbc.M203845200
Ralle M, Cooper MJ, Lutsenko S, Blackburn NJ (1998) The Menkes disease protein binds copper via novel 2-coordinate Cu(I)-cysteinates in the N-terminal domain. J Am Chem Soc 120(51):13525–13526
Ralle M, Lutsenko S, Blackburn NJ (2003) X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J Biol Chem 278(25):23163–23170
Ralle M, Lutsenko S, Blackburn NJ (2004) Copper transfer to the N-terminal domain of the Wilson disease protein (ATP7B): X-ray absorption spectroscopy of reconstituted and chaperone-loaded metal binding domains and their interaction with exogenous ligands. J Inorg Biochem 98(5):765–774
McRae R, Lai B, Fahrni CJ (2010) Copper redistribution in Atox1-deficient mouse fibroblast cells. J Biol Inorg Chem 15(1):99–105. doi:10.1007/s00775-009-0598-1
Goodman L (1953) Alzheimer's disease; a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J Nerv Ment Dis 118(2):97–130
Bonda DJ, Lee HG, Blair JA, Zhu X, Perry G, Smith MA (2011) Role of metal dyshomeostasis in Alzheimer's disease. Metallomics 3(3):267–270. doi:10.1039/c0mt00074d
Hung YH, Bush AI, Cherny RA (2010) Copper in the brain and Alzheimer's disease. J Biol Inorg Chem 15(1):61–76. doi:10.1007/s00775-009-0600-y
Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling KQ, Huang X, Moir RD, Wang D, Sayre LM, Smith MA, Chen SG, Bush AI (2004) Copper mediates dityrosine cross-linking of Alzheimer's amyloid-beta. Biochemistry 43(2):560–568. doi:10.1021/bi0358824
Huang X, Atwood CS, Moir RD, Hartshorn MA, Tanzi RE, Bush AI (2004) Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer's Abeta peptides. J Biol Inorg Chem 9(8):954–960. doi:10.1007/s00775-004-0602-8
Zhu X, Su B, Wang X, Smith MA, Perry G (2007) Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci 64(17):2202–2210. doi:10.1007/s00018-007-7218-4
S chrag M, Mueller C, Oyoyo U, Smith MA, Kirsch WM (2011) Iron, zinc and copper in the Alzheimer's disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol 94(3):296–306. doi:10.1016/j.pneurobio.2011.05.001
Bush AI (2000) Metals and neuroscience. Curr Opin Chem Biol 4(2):184–191
Tougu V, Tiiman A, Palumaa P (2011) Interactions of Zn(II) and Cu(II) ions with Alzheimer's amyloid-beta peptide. Metal ion binding, contribution to fibrillization and toxicity. Metallomics 3(3):250–261. doi:10.1039/c0mt00073f
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci 158(1):47–52
Hutchinson RW, Cox AG, McLeod CW, Marshall PS, Harper A, Dawson EL, Howlett DR (2005) Imaging and spatial distribution of beta-amyloid peptide and metal ions in Alzheimer's plaques by laser ablation-inductively coupled plasma-mass spectrometry. Anal Biochem 346(2):225–233. doi:10.1016/j.ab.2005.08.024
Rajendran R, Minqin R, Ynsa MD, Casadesus G, Smith MA, Perry G, Halliwell B, Watt F (2009) A novel approach to the identification and quantitative elemental analysis of amyloid deposits–insights into the pathology of Alzheimer's disease. Biochem Biophys Res Commun 382(1):91–95. doi:10.1016/j.bbrc.2009.02.136
Wang H, Wang M, Wang B, Li M, Chen H, Yu X, Zhao Y, Feng W, Chai Z (2012) The distribution profile and oxidation states of biometals in APP transgenic mouse brain: dyshomeostasis with age and as a function of the development of Alzheimer's disease. Metallomics 4(3):289–296. doi:10.1039/c2mt00104g
Acknowledgments
The authors gratefully acknowledge the use of the facilities at the Advanced Photon Source. This work was supported by the National Institutes of Health grant GM090016 to M.R. The use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science contract DE-AC-02-6CH11357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Metallomics with guest editors Uwe Karst and Michael Sperling.
Rights and permissions
About this article
Cite this article
Vogt, S., Ralle, M. Opportunities in multidimensional trace metal imaging: taking copper-associated disease research to the next level. Anal Bioanal Chem 405, 1809–1820 (2013). https://doi.org/10.1007/s00216-012-6437-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6437-1