Abstract
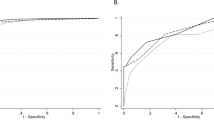
A number of metabolic abnormalities have been observed in pregnancies complicated by intrauterine growth restriction (IUGR). Metabolic fingerprinting and clinical metabolomics have recently been proposed as tools to investigate individual phenotypes beyond genomes and proteomes and to advance hypotheses on the genesis of diseases. Non-targeted metabolomic profiling was employed to study fetal and/or placental metabolism alterations in IUGR fetuses by liquid chromatography high-resolution mass spectrometry (LC-HRMS) analysis of cord blood collected soon after birth. Samples were collected from 22 IUGR and 21 appropriate for gestational age (AGA) fetuses. Birth weight differed significantly between IUGR and AGA fetuses (p < 0.001). Serum samples were immediately obtained and deproteinized by mixing with methanol at room temperature and centrifugation; supernatants were lyophilized and reconstituted in water for analysis. LC-HRMS analyses were performed on an Orbitrap mass spectrometer linked to a Surveyor Plus LC. Samples were injected into a 1.0 × 150-mm Luna C18 column. Spectra were collected in full-scan mode at a resolution of approximately 30,000. Data were acquired over the m/z range of 50–1,000, with measurements performed in duplicate. To observe metabolic variations between the two sets of samples, LC-HRMS data were analyzed by a principal component analysis model. Many features (e.g., ionic species with specific retention times) differed between the two classes of samples: among these, the essential amino acids phenylalanine, tryptophan, and methionine were identified by comparison with available databases. Logistic regression coupled to a receiver-operating characteristic curve identified a cut-off value for phenylalanine and tryptophan, which gave excellent discrimination between IUGR and AGA fetuses. Non-targeted LC-HRMS analysis of cord blood collected at birth allowed the identification of significant differences in relative abundances of essential amino acids between IUGR and AGA fetuses, emerging as a promising tool for studying metabolic alterations.





Similar content being viewed by others
References
Chauhan SP, Gupta LM, Hendrix NW, Berghella V (2009) Intrauterine growth restriction: comparison of American College of Obstetricians and Gynecologists practice bulletin with other national guidelines. Am J Obstet Gynecol 200(4):e401–e406. doi:10.1016/j.ajog.2008.11.025, 409
Barker DJ (2006) Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49(2):270–283
Barker DJ (2000) In utero programming of cardiovascular disease. Theriogenology 53(2):555–574
Baschat AA, Viscardi RM, Hussey-Gardner B, Hashmi N, Harman C (2009) Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet Gynecol 33(1):44–50. doi:10.1002/uog.6286
Cosmi E, Visentin S, Fanelli T, Mautone AJ, Zanardo V (2009) Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet Gynecol 114(5):1109–1114. doi:10.1097/AOG.0b013e3181bb23d300006250-200911000-00022
Miller J, Turan S, Baschat AA (2008) Fetal growth restriction. Semin Perinatol 32(4):274–280. doi:10.1053/j.semperi.2008.04.010
Baschat AA (2010) Fetal growth restriction—from observation to intervention. J Perinat Med 38(3):239–246. doi:10.1515/JPM.2010.041
Lucio M, Fekete A, Weigert C, Wagele B, Zhao X, Chen J, Fritsche A, Haring HU, Schleicher ED, Xu G, Schmitt-Kopplin P, Lehmann R (2010) Insulin sensitivity is reflected by characteristic metabolic fingerprints—a Fourier transform mass spectrometric non-targeted metabolomics approach. PLoS One 5(10):e13317. doi:10.1371/journal.pone.0013317
Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, Shockcor J, Holmes E, Nicholson JK (2010) Global metabolic profiling procedures for urine using UPLC-MS. Nat Protoc 5(6):1005–1018
Antonucci R, Atzori L, Barberini L, Fanos V (2010) Metabolomics: the “new clinical chemistry” for personalized neonatal medicine. Minerva Pediatr 62(3 Suppl 1):145–148
König S (2011) Urine molecular profiling distinguishes health and disease: new ways in diagnostics? Test case UPLC-MS. Exp Rev Mol Diagn 11(4):383–391
Pardi G, Marconi AM, Cetin I (2002) Placental–fetal interrelationship in IUGR fetuses—a review. Placenta 23(Suppl A):S136–S141. doi:10.1053/plac.2002.0802
Paladini D, Rustico M, Viora E, Giani U, Bruzzese D, Campogrande M, Martinelli P (2005) Fetal size charts for the Italian population. Normative curves of head, abdomen and long bones. Prenat Diagn 25(6):456–464. doi:10.1002/pd.1158
Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G (2006) XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem 78(3):779–787. doi:10.1021/ac051437y
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5(10):R80. doi:10.1186/gb-2004-5-10-r80
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95(25):14863–14868
Horgan RP, Broadhurst DI, Dunn WB, Brown M, Heazell AE, Kell DB, Baker PN, Kenny LC (2010) Changes in the metabolic footprint of placental explant-conditioned medium cultured in different oxygen tensions from placentas of small for gestational age and normal pregnancies. Placenta 31(10):893–901. doi:10.1016/j.placenta.2010.07.002
Dunn WA, Broadhurst D, Brown M, Baker PN, Redman CWG, Kenny LC, Kell DB (2008) Metabolic profiling of serum using ultra performance liquid chromatography and the LTQ-Orbitrap mass spectrometry system. J Chromatogr B 871:288–298
Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC (2001) Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 86(11):5427–5432
Baioria R, Sooranna SR, Ward S, Hancock M (2002) Placenta as a link between amino acids, insulin-IGF axis, and low birth weight: evidence from twin studies. J Clin Endocrinol Metab 87(1):308–315
Morris NH, Burston D, Ramsay B, Sooranna SR (1995) Free amino acid concentrations in normal and abnormal third trimester placental villi. Eur J Clin Invest 25(10):796–798
Chien PF, Smith K, Watt PW, Scrimgeour CM, Taylor DJ, Rennie MJ (1993) Protein turnover in the human fetus studied at term using stable isotope tracer amino acids. Am J Physiol 265(1 Pt 1):E31–E35
Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC (1990) Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162(1):253–261
De Boo HA, Van Zijl PL, Smith DE, Kulik W, Lafeber HN, Harding JE (2005) Arginine and mixed amino acids increase protein accretion in the growth-restricted and normal ovine fetus by different mechanisms. Pediatr Res 58(2):270–277. doi:10.1203/01.PDR.0000169977.48609.55
Bauer R, Walter B, Vorwieger G, Bergmann R, Fuchtner F, Brust P (2001) Intrauterine growth restriction induces up-regulation of cerebral aromatic amino acid decarboxylase activity in newborn piglets: [18F]fluorodopa positron emission tomographis study. Pediatr Res 49(4):474–480
Matsueda S, Niiyama Y (1982) The effects of excess amino acids on maintenance of pregnancy and fetal growth in rats. J Nutr Sci Vitaminol (Tokyo) 28(5):557–573
Hernandez RJ, Manjarrez GG, Chagoya G (1989) Newborn humans and rats malnourished in utero: free plasma l-tryptophan, neutral amino acid and brain serotonin synthesis. Brain Res 488:1–13
Hernandez-Rodriguez J, Meneses L, Herrera R, Manjarrez G (2009) Another abnormal trait in the serotonin metabolism path in intrauterine growth-restricted infants. Neonatology 95(2):125–131. doi:10.1159/000153096
Manjarrez G, Cisneros I, Herrera R, Vazquez F, Robles A, Hernandez J (2005) Prenatal impairment of brain serotonergic transmission in infants. J Pediatr 147(5):592–596. doi:10.1016/j.jpeds.2005.06.025
Huang WQ, Zhang CL, Di XY, Zhang RQ (1998) Studies on the localization of 5-hydroxytryptamine and its receptors in human placenta. Placenta 19(8):655–661
Dunn WB, Brown M, Worton SA, Crocker IP, Broadhurst D, Horgan R, Kenny LC, Baker PN, Kell DB, Heazell AE (2009) Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta 30(11):974–980. doi:10.1016/j.placenta.2009.08.008
Aubard Y, Darodes N, Cantaloube M (2000) Hyperhomocysteinemia and pregnancy—review of our present understanding and therapeutic implications. Eur J Obstet Gynecol Reprod Biol 93(2):157–165
Moores RR Jr, Vaughn PR, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G (1994) Glutamate metabolism in fetus and placenta of late-gestation sheep. Am J Physiol 267(1 Pt 2):R89–R96
Matthews JC, Beveridge MJ, Malandro MS, Rothstein JD, Campbell-Thompson M, Verlander JW, Kilberg MS, Novak DA (1998) Activity and protein localization of multiple glutamate transporters in gestation day 14 vs. day 20 rat placenta. Am J Physiol 274(3 Pt 1):C603–C614
Bell AW, Hay WW Jr, Ehrhardt RA (1999) Placental transport of nutrients and its implications for fetal growth. J Reprod Fertil Suppl 54:401–410
Grosso LM, Triche EW, Belanger K, Benowitz NL, Holford TR, Bracken MB (2006) Caffeine metabolites in umbilical cord blood, cytochrome P-450 1A2 activity, and intrauterine growth restriction. Am J Epidemiol 163(11):1035–1041. doi:10.1093/aje/kwj125
Jobgen WS, Ford SP, Jobgen SC, Feng CP, Hess BW, Nathanielsz PW, Li P, Wu G (2008) Baggs ewes adapt to maternal undernutrition and maintain conceptus growth by maintaining fetal plasma concentrations of amino acids. J Anim Sci 86(4):820–826. doi:10.2527/jas.2007-0624
Kwon H, Ford SP, Bazer FW, Spencer TE, Nathanielsz PW, Nijland MJ, Hess BW, Wu G (2004) Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol Reprod 71(3):901–908. doi:10.1095/biolreprod.104.029645biolreprod.104.029645
Wu G, Bazer FW, Datta S, Johnson GA, Li P, Satterfield MC, Spencer TE (2008) Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids 35(4):691–702. doi:10.1007/s00726-008-0052-7
Cetin I, Alvino G (2009) Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30(Suppl A):S77–S82. doi:10.1016/j.placenta.2008.12.006
Acknowledgments
This study was financed by “Progetto d’Ateneo 2009” no. CPDA098835 from the University of Padova.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.20 mb)
Rights and permissions
About this article
Cite this article
Favretto, D., Cosmi, E., Ragazzi, E. et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem 402, 1109–1121 (2012). https://doi.org/10.1007/s00216-011-5540-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5540-z