Abstract
Following hemorrhage-causing injury, lactate levels rise and correlate with the severity of injury and are a surrogate of oxygen debt. Posttraumatic injury also includes hyperglycemia, with continuously elevated glucose levels leading to extensive tissue damage, septicemia, and multiple organ dysfunction syndrome. A temporary, implantable, integrated glucose and lactate biosensor and communications biochip for physiological status monitoring during hemorrhage and for intensive care unit stays has been developed. The dual responsive, amperometric biotransducer uses the microdisc electrode array format upon which were separately immobilized glucose oxidase and lactate oxidase within biorecognition layers, 1.0–5.0 μm thick, of 3 mol% tetraethyleneglycol diacrylate cross-linked p(HEMA-co-PEGMA-co-HMMA-co-SPA)-p(Py-co-PyBA) electroconductive hydrogels. The device was then coated with a bioactive hydrogel layer containing phosphoryl choline and polyethylene glycol pendant moieties [p(HEMA-co-PEGMA-co-HMMA-co-MPC)] for indwelling biocompatibility. In vitro cell proliferation and viability studies confirmed both polymers to be non-cytotoxic; however, PPy-based electroconductive hydrogels showed greater RMS 13 and PC12 proliferation compared to controls. The glucose and lactate biotransducers exhibited linear dynamic ranges of 0.10–13.0 mM glucose and 1.0–7.0 mM and response times (t 95) of 50 and 35–40 s, respectively. Operational stability gave 80% of the initial biosensor response after 5 days of continuous operation at 37 °C. Preliminary in vivo studies in a Sprague–Dawley hemorrhage model showed tissue lactate levels to rise more rapidly than systematic lactate. The potential for an implantable biochip that supports telemetric reporting of intramuscular lactate and glucose levels allows the refinement of resuscitation approaches for civilian and combat trauma victims.

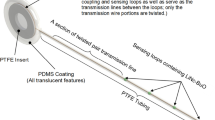
Schematic of an electrode-supported, two-layer hydrogel membrane for bioreceptor hosting and tissue biocompatibility










Similar content being viewed by others
References
Meislin H et al (1997) Fatal trauma: the modal distribution of time to death is a function of patient demographics and regional resources. The Journal of Trauma: Injury, Infection, and Critical Care 43(3):433–440
Edgar CE (2009) Baghdad ER revisited: The 28th Combat Support Hospital of Operation Iraqi Freedom 2006–2008. In: Tountas KH (ed) Third Military Health Research Forum (MHRF). Hallmark Crown Center, Kansas City, Missouri, USA
Sambasivan CN, Schreiber MA (2009) Emerging therapies in traumatic hemorrhage control. Curr Opin Crit Care 15:560–568
Vincent JL (1998) Lactate and biochemical indexes of oxygenation. In: Tobin MJ (ed) Principles and practice of intensive care monitoring. McGraw-Hill, New York
Ward KR, Ivatury RR, Barbee RW (2001) Endpoints of resuscitation for the victim of trauma. J Intensive Care Med 16:55–75
Convertino VA et al (2008) Physiological and medical monitoring for en route care of combat casualties. The Journal of Trauma: Injury, Infection, and Critical Care 64(4):S342–S353
Gunnerson KJ et al (2008) Pre-hospital point of care lactate does not corelate with initial hemodynamic variables. Chest 134:p65003
Huckabee WE (1963) Lactic acidosis. Am J Cardiol 12:663–666
Broder G, Weil MH (1964) Excess lactate: an index of reversibility of shock in human patients. Science 143:1457–1459
Vitek V, Cowley RA (1971) Blood lactate in the prognosis of various forms of shock. Ann Surg 173(2):308–313
Bakker J et al (1996) Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 171(2):221–226
Schuster H-P (1984) Prognostic value of blood lactate in critically ill patients. Resuscitation 11(3–4):141–146
Lee SW et al (2008) Lactic acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg Med J 25(10):659–665
Gunnerson KJ et al (2006) Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Critical Care 10:R22
Gunnerson KJ (2005) Clinical review: the meaning of acid–base abnormalities in the intensive care unit—epidemiology. Crit Care 9(5):508–516
Dunham CM et al (1991) Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock. Crit Care Med 19(2):231–243
Siegel JH et al (1990) Early physiologic predictors of injury severity and death in blunt multiple trauma. Arch Surg 125(4):498–508
Andersen BJ et al (1988) Effect of posttraumatic hypoventilation on cerebral energy metabolism. J Neurosurg 68:601–607
Schurr A et al (1997) Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res 744:105–111
Chen T et al (2000) Lactate/glucose dynamics after rat fluid percussion brain injury. J Neurotrauma 17:135–142
Aduen J et al (1994) The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA 272(21):1678–1685
Rady MY (1996) Triage of critically ill patients: an overview of interventions. Emerg Med Clin North Am 14(1):13–33
Jain KK, Fisher B (1989) Oxygen uptake, transport and utilization in the human body. In: Jain KK, Fischer B (eds) Oxygen in physiology and medicine. Charles C. Thomas Pub Ltd, Springfield IL, pp 25–53, 367 pp
Raedler C et al (2004) Treatment of uncontrolled hemorrhagic shock after liver trauma: fatal effects of fluid resuscitation versus improved outcome after vasopressin. Anesth Analg 98(6):1759–1766
Huang Y-CT (2005) Monitoring oxygen delivery in the critically ill. Chest 128(5 suppl 2):554S–560S
Grissom CKW, Lindell K, Clemmer TP, Morris AH (2006) Theoretical advantage of oxygen treatment for combat casualties during medical evacuation at high altitude. The Journal of Trauma: Injury, Infection, and Critical Care 61(2):461–467
Antonelli M, Levy M, Andrews P, Chastre J, Hudson L, Manthous C, Meduri G, Moreno R, Putensen C, Stewart T, Torres A (2007) Hemodynamic monitoring in shock and implications for management: International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med 33(4):575–590
Vincent JL et al (1983) Serial lactate determinations during circulatory shock. Crit Care Med 11:449–451
van den Berghe G et al (2001) Intensive insulin therapy in the critically ill patients. N Engl J Med 345(19):1359–1367
van den Berghe G et al (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354(5):449–461
Finfer S et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360(13):1283–1297
Atanasova P et al (1997) Implantation of a refillable glucose monitoring-telemetry device. Biosens Bioelectron 12(7):669–680
Valdastri P et al (2008) An implantable ZigBee ready telemetric platform for in vivo monitoring of physiological parameters. Sens Actuators, A, Phys 142(1):369–378
Receveur RAM, Lindemans FW, Rooij NFd (2007) Microsystem technologies for implantable applications. J Micromech Microeng 17(5):R50
Ativanichayaphong T et al (2008) An implantable, wireless and batteryless impedance sensor capsule for detecting acidic and non-acidic reflux. Gastroenterology 134(4):A-63
Lvovich VF, Liu CC, Smiechowski MF (2006) Optimization and fabrication of planar interdigitated impedance sensors for highly resistive non-aqueous industrial fluids. Sens Actuators, B, Chem 119(2):490–496
Schulman JH et al. (2005) Implantable enzyme-based monitoring system having improved longevity due to improved exterior surfaces. Free Patents Online, USPTO, editor. 2005-08-23, Alfred E. Mann Foundation for Scientific Research, USA
Mann AE et al. (2009) Telemetered characteristic monitor system and method of using the same. Free Patents Online, USPTO, editor. 2009-10-13, Medtronic Minimed, Inc., USA
Atanasov P et al (1996) Short-term canine implantation of a glucose monitoring-telemetry device. Med Eng Phys 18(8):632–640
Ahmadi MM, Jullien GA (2009) A wireless-implantable microsystem for continuous blood glucose monitoring. IEEE Trans Biomed Circuits Syst 3(3):169–180
Tathireddy P et al (2009) Implantable microsystems and neuro electronic interfaces. IET Digest 2009(12923):8
Guiseppi-Elie A, Brahim S, Wilson A (2006) Biosensors based on electrically conducting polymers. In: Skotheim T, Reynolds JR (eds) Handbook of conducting polymers: conjugated polymer processing and applications, 3rd edn. Marcel Dekker, New York, pp 435–479
Wang L et al. (2002) An integrated sensor microsystem for industrial and biomedical applications. IEEE Instrumentation and Measurement Technology Conference, pp 1717–1720
Cheney CP et al (2007) In vivo ethanol vapor detection in the interstitial fluid of a Wistar rat using piezoresistive microcantilevers. Appl Phys Lett 90(90):013901
Rahman AR, Justin G, Guiseppi-Elie A (2009) Towards an implantable biochip for glucose and lactate monitoring using microdisc electrode arrays (MDEAs). Biomedical Microdevices: BioMEMS and Biomedical NanoTechnology Biomedical Microdevices 11(1):75–85
Guiseppi-Elie A et al (2005) Design of a subcutaneous implantable biochip for monitoring of glucose and lactate. IEEE Sens J 5(3):345–355
Rahman ARA et al (2009) Fabrication and packaging of a dual sensing electrochemical biotransducer for glucose and lactate useful in intramuscular physiologic status monitoring. IEEE Sens J 9(12):1856–1863
Abraham S et al (2005) Molecularly engineered p(HEMA)-based hydrogels for implant biochip biocompatibility. Biomaterials 26:4767–4778
Guiseppi-Elie A (2010) Electroconductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials 31(10):2701–2716
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108(2):814–825
Leach JB et al (2004) Development of photocrosslinked hyaluronic acid–polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res 70A:74–82
Yu B et al (2008) Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens Bioelectron 23:1278–1284
Yang Y et al (2004) Phosphorylcholine-containing polymers for use in cell encapsulation. Artificial Cells, Blood Substitutes, and Biotechnology 32(1):91–104
Patel JD et al (2005) Phospholipid polymer surfaces reduce bacteria and leukocyte adhesion under dynamic flow conditions. J Biomed Mater Res A 73(3):359–366
Konopka SJ, McDuffie B (1970) Diffusion coefficients of ferricyanide and ferrocyanide ions in aqueous media using twin electrode thin layer electrochemistry. Anal Chem 42(14):1741–1746
Nederberg F et al (2003) Phosphoryl choline introduces dual activity in biomimetic ionomers. Macromolecules 36(22):8440–8448
Tang Y et al (2003) Interfacial structure of phosphorylcholine incorporated biocompatible polymer films. Macromolecules 36(22):8440–8448
Lyu S, Untereker D (2009) Degradability of polymers for implantable biomedical devices. Int J Mol Sci 10(9):4033–4065
Zheng J, He Y, Chen S, Li L, Bernards MT, Jiang S (2006) Molecular simulation studies of the structure of phosphorylcholine self-assembled monolayers. J Chem Phys 125(17):47141–47147
Berglin M, Andersson M, Elwing ASH (2004) The effect of substrate molecular mobility on surface induced immune complement activation and blood plasma coagulation. Biomaterials 25(19):4581–4590
Abraham S, Brahim S, Guiseppi-Elie A (2004) Molecularly engineered hydrogels for implant biocompatibility. IEEE Engineering in Medicine and Biology Society. IEMBS ‘04. IEEE, San Francisco, California, USA
Abraham S, Guiseppi-Elie A (2005) Molecularly engineered hydrogels possessing poly(ethyleneglycol) and phosphorylcholine for implant biocompatibility. IEEE Engineering in Medicne and Biology Society. IEEE-EMBS 2005. IEEE, Shanghai, China
Xu J et al (2003) Ozone-induced grafting phosphorylcholine polymer onto silicone film grafting 2-methacryloyloxyethyl phosphorylcholine onto silicone film to improve hemocompatibility. Colloids Surf, B Biointerfaces 30(3):215–223
Justin G, Guiseppi-Elie A (2010) An electroconductive blend of p(HEMA-co-PEGMA-co-HMMA-co-SPMA) hydrogels and p(Py-co-PyBA): in vitro biocompatibility. J Bioact Compat Polym 25(2):121–140
Rahman ARA, Justin G, Guisepp-Elie A (2009) Bioactive hydrogel layers on microdisc electrode arrays: impedimetric characterization and equivalent circuit modeling. Electroanalysis 21(10):1125–1134
Justin G, Abdur Rahman AR, Guisepp-Elie A (2009) Bioactive hydrogel layers on microdisc electrode arrays: cyclic voltammetry experiments and simulations. Electroanalysis 21(10):1125–1134
Justin G, Guiseppi-Elie A (2009) Characterization of electroconductive blends of p(HEMA-co-PEGMA-co-HMMA-co-SPMA) hydrogels and p(Py-co-PyBA). Biomacromolecules 10(9):2539–2549
Brahim S, Narinesingh D, Guiseppi-Elie A (2002) Interferent suppression using a novel polypyrrole-containing hydrogel in amperometric enzyme biosensors. Electroanalysis 14(9):627–633
Schaefer M et al (2002) The complex dielectric spectrum of heart tissue during ischemia. Bioelectrochemistry 58(2):171–180
Seone F (2004) Brain electrical impedance at various frequencies: the effect of hypoxia. Paper presented at the 26th Anuual Conference of the IEEE Engineering in Medicine and Biology Society
Chertow GM et al (1997) Bioimpedance norms for the hemodialysis population. Kidney Int 52(6):1617–1621
Perramon D (2007) In vivo detection of liver steatosis in rats based on impedance spectroscopy. Physiol Meas 28:813–828
Gheorghiu M, Gersing E, Gheorghiu E (2006) Quantitative analysis of impedance spectra of organs during ischemia. Ann N Y Acad Sci (Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology) 873:65–71
Tarulli AW et al (2006) Electrical impedance in bovine skeletal muscle as a model for the study of neuromuscular disease. Physiol Meas 27:1269–1279
Cornish BH, Thomas BJ, Ward LC (1998) Effect of temperature and sweating on bioimpedance measurements. Appl Radiat Isotopes 49(5/6):475–476
Chauveau N et al (1999) Ex-vivo discrimination between normal and pathological tissues in human breast surgical biopsies using bioimpedance spectroscopy. Ann NY Acad Sci 873(1):42–50
Toso S et al (2000) Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition 16(2):120–124
Green RA et al (2010) Conducting polymer-hydrogels for medical electrode applications. Sci Technol Adv Mater 11(1):014107
Lu X, Yang J, Zhao JB, Gregersen H, Kassab GS (2003) Shear modulus of porcine coronary artery: contributions of media and adventitia. Am J Physiol Heart Circ Physiol 285:1966–1975
Mathur AB et al (2001) Endothelial, cardiac muscle, and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J Biomech 34:1545–1553
Nicolle S et al (2005) Shear linear behavior of brain tissue over a large frequency range. Biorheology 42:209–223
Rabinovich Y et al (2005) Atomic force microscopy measurement of the elastic properties of the kidney epithelial cells. J Colloid Interface Sci 285:125–135
Liu Z, Bilston L (2000) On the viscoelastic character of liver tissue: experiments and modelling of the linear behaviour. Biorheology 37(3):191–201
Liao D et al (2003) The effect of epidermal growth factor on the incremental Young’s moduli in the rat small intestine. Mechanical Engineering & Physics 25:413–418
Ogneva IV, Lebedev DV, Shenkman BS (2010) Transversal stiffness and Young’s modulus of single fibers from rat soleus muscle probed by atomic force microscopy. Biophys J 98:418–424
Holt B, Tripathi A, Morgan J (2008) Viscoelastic response of human skin to low magnitude physiologically relevant shear. J Biomech 41(12):2689–2695
Park E, Maniatty AM (2006) Shear modulus reconstruction in dynamic elastography: time harmonic case. Phys Med Biol 51:3697–3721
Alcarez J (2003) Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J 84:2071–2079
Stammen JA et al (2001) Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 22:799–806
Muratoglu OK et al (2009) PVA hydrogels: methods of making covalently crosslinked vinyl polymer hydrogels having advantageous physical properties. Patentdocs, U.S.P. Office, editor, USA
Meakin JR et al (2003) Rheological properties of poly(2-hydroxyethyl methacrylate) (pHEMA) as a function of water content and deformation frequency. J Mater Sci Mater Med 14:783–787
Farahi RH et al. (2007) Integrated electronics platforms for wireless implantable biosensors. Life Science Systems and Applications Workshop 2007. IEEE/NIH, pp 27–30
Xu S et al (2008) Electrochemical response of ferrocenium filled calix[4]arene capsules in solution and immobilized on gold. Electrochim Acta 53(27):7981–7987
Bean LS et al (2005) Photocurable ferrocene-containing poly(2-hydroxyl ethyl methacrylate) films for mediated amperometric glucose biosensor. Thin Solid Films 477(1–2):104–110
Boztas A, Guiseppi-Elie A (2009) Immobilization and release of the redox mediator ferrocene monocarboxylic acid from within cross-linked p(HEMA-co-PEGMA-co-HMMA) hydrogels. Biomacromolecules 10(8):2135–2143
Qvisth V et al (2008) Catecholamine regulation of local lactate production in vivo in skeletal muscle and adipose tissue: role of β-adrenoreceptor subtypes. J Clin Endocrinol Metab 93(1):240–246
Corstjens AM et al (2006) Accuracy and feasibility of point-of-care and continuous blood glucose analyzing in critically ill ICU patients. Crit Care 10(5):R135
Hylton DM, Shalaby SW, Latour RA Jr (2005) Direct correlation between adsorption-induced changes in protein structure and platelet adhesion. J Biomed Materi Res Part A 73A(3):349–358
Timmer BH et al. (2001) Planar interdigitated conductivity sensors for low electrolyte concentrations. Proceedings Semiconductor Sensor and Actuator Technology, SeSens, Veldhoven, Utrecht, STW, pp 878–883
Yang X, Zhang G (2007) The voltammetric performance of interdigitated electrodes with different electron-transfer rate constants. Sens Actuators, B 126(2):624–631
Olthuis W, Streekstra W, Bergveld P (1995) Theoretical and experimental determination of cell constants of planar-interdigitated electrolyte conductivity sensors. Sens Actuators, B, Chem 24(1–3):252–256
Wichterle O, Lim D (1960) Hydrophilic gels in biologic use. Nature 185:117
Mabilleau G et al (2006) Effects of the length of crosslink chain on poly(2-hydroxyethyl methacrylate) (pHEMA) swelling and biomechanical properties. J Biomed Mater Res A 77(1):35–42
Guiseppi-Elie A, Lesho MJ, Sheppard NF Jr (1998) Electrical impedance properties of chemically responsive hydrogels. In: Wise DL et al (eds) Electrical and optical polymer systems, fundamentals, methods, and applications. Marcel Dekker, New York, pp 1187–1211
Kaji H et al (2010) Electrically induced contraction of C2C12 myotubes cultured on a porous membrane-based substrate with muscle tissue-like stiffness. Biomaterials 31(27):6981–6986
Mamishev AV, Lesieutre BC, Zahn M (1997) Parameter estimation using an interdigital dielectrometry sensor with finite-element software. Conference on Electrical Insulation and Dielectric Phenomena, C.o.E.I.a.D. Phenomena, editor., IEEE Minneapolis, Minnesota
Acknowledgments
This work was supported by the US Department of Defense (DoDPRMRP) grant PR023081/DAMD17-03-1-0172, by the Consortium of the Clemson University Center for Bioelectronics, Biosensors and Biochips (C3B), and by ABTECH Scientific, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guiseppi-Elie, A. An implantable biochip to influence patient outcomes following trauma-induced hemorrhage. Anal Bioanal Chem 399, 403–419 (2011). https://doi.org/10.1007/s00216-010-4271-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4271-x