Abstract
Rationale
Tramadol is a prescription analgesic that activates mu opioid and monoamine receptor systems. Tramadol is thought to have limited abuse potential compared to mu opioid agonists, but laboratory data indicate that it shares some of their pharmacodynamic effects.
Objectives
This study evaluated the effect of mu opioid receptor blockade with naltrexone on the pharmacodynamic action of tramadol in humans.
Methods
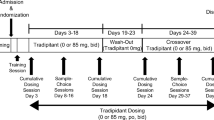
This inpatient, double-blind, randomized, within-subject study examined the effects of oral placebo, tramadol (87.5, 175, and 350 mg), and hydromorphone (4 and 16 mg; positive control) after 1 h pretreatment with oral naltrexone (0 and 50 mg). Ten recreational opioid users completed the study. Pharmacodynamic effects were measured before and for 7 h after initial drug administration.
Results
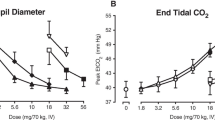
Lower doses of tramadol and hydromorphone were generally placebo-like. Hydromorphone (16 mg) produced prototypic mu opioid agonist-like effects that were blocked by naltrexone. Tramadol (350 mg) produced miosis and increased ratings of “Good Effects” and “Liking” but also increased ratings of “Bad Effects.” Naltrexone reversed tramadol-induced physiological effects and mydriasis emerged, but unlike results with hydromorphone, naltrexone only partially attenuated tramadol’s positive subjective effects and actually enhanced several unpleasant subjective ratings.
Conclusions
Naltrexone can be used to disentangle the mixed neuropharmacological actions of tramadol. High-dose tramadol produces a mixed profile of effects. These data suggest that both mu and non-mu opioid actions play a role in tramadol’s subjective profile of action.


Similar content being viewed by others
References
Ananthan S, Kezar HS III, Carter RL et al (1999) Synthesis, opioid receptor binding and biological activities of naltrexone-derived pyrido- and pyrimidomorphinans. J Med Chem 42:3527–3538
Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL (2006) Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp Clin Psychopharmacol 14:109–120
Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Muñoz A (2005) Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf 14:851–859
Daws LC (2009) Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther 121:89–99
Driessen B, Reimann W, Giertz H (1993) Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol 108:806–811
Duke AN, Bigelow GE, Lanier RK, Strain EC (2011) Discriminative stimulus effects of tramadol in humans. J Pharmacol Exp Ther 338:255–262
Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML (1996) Buprenorphine’s physical dependence potential: antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther 276:449–459
Epstein DH, Preston KL, Jasinski DR (2006) Abuse liability, behavioral pharmacology and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol Psychol 73:90–99
Filip M, Wydra K, Inan SY, Dziedzicka-Wasylewska M, Przegalinski E (2004) Opioid and monoamine systems mediate the discriminative stimulus of tramadol in rats. Eur J Pharmacol 498:143–151
Frink MC, Hennies HH, Englberger W, Haurand M, Wilffert B (1996) Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittelforschung 46:1029–1036
Gillen C, Haurand M, Kobelt DJ, Wnendt S (2000) Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol 362:116–121
Grond S, Sablotzki A (2004) Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923
Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE (2002) Comparison of the subjective, physiological and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 67:149–156
Ide S, Minami M, Ishihara K, Uhl GR, Sora I, Ikeda K (2006) Mu opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacology 51:651–658
Jasinski DR, Preston K, Sullivan JT, Testa MP (1993) Abuse potential of oral tramadol. NIDA Res Monogr 132:103
Jasinski DR, Faries DE, Moore RJ, Schuh LM, Allen AJ (2008) Abuse liability assessment of atomoxetine in a drug-abusing population. Drug Alcohol Depend 95:140–146
Laeng B, Sirois S, Gredebäck G (2012) Pupillometry: a window into the preconscious? Perspect Psychol Sci 7:18–27
Lai J, Ma S-w, Porreca F, Raffa (1996) Tramadol, M1 metabolite and enantiomer affinities for cloned human opioid receptors expressed in transfected HN9.10 neuroblastoma cells. Eur J Pharmacol 316:369–372
Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC (2010) Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology (Berl) 211:457–466
Lee MC, Wagner HN Jr, Tanada S, Frost JJ, Bice AN, Dannals RF (1988) Duration of occupancy of opiate receptors by naltrexone. J Nucl Med 29:1207–1211
Liao S, Hill JF, Nayak RK (1992) Pharmacokinetics of tramadol following single and multiple oral doses in man. Pharm Res 9(Suppl):308
Lintz W, Barth H, Osterloh G, Schmidt-Bothelt E (1986) Bioavailability of enteral tramadol formulations. 1st communication: capsules. Arzneimittelforschung 36:1278–1283
Lofwall MR, Walsh SL, Bigelow GE, Strain EC (2007) Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology 194:381–393
Manchikanti L, Fellows B, Ailinani H, Pampati V (2010) Therapeutic use, abuse and nonmedical use of opioids: A ten-year perspective. Pain Physician 13:401–435
Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE (1976) The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197:517–532
Martin CA, Guenthner G, Bingcang C, Rayens MK, Kelly TH (2007) Measurement of the subjective effects of methylphenidate in 11- to 15-year-old children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 17:63–73
Matouskova O, Slanar O, Chytil L, Perlik F (2011) Pupillometry in healthy volunteers as a biomarker of tramadol efficacy. J Clin Pharm Ther 36:513–517
Matthiesen T, Wohrmann T, Coogan TP, Uragg H (1998) The experimental toxicology of tramadol: an overview. Toxicol Lett 95:63–71
McLeod D, Griffiths RR, Bigelow GE, Yingling J (1982) An automated version of the digit symbol substitution test (DSST). Behav Res Methods Instrum 14:433–436
Mello NK, Mendelson JH, Bree MP (1981) Naltrexone effects on morphine and food self-administration in morphine-dependent rhesus monkeys. J Pharmacol Exp Ther 218:550–557
O’Connor EC, Mead AN (2010) Tramadol acts as a weak reinforcer in the rat self-administration model, consistent with its low abuse liability in humans. Pharmacol Biochem Behav 96:279–286
Preston KL, Bigelow GE (1993) Differential naltrexone antagonism of hydromorphone and pentazocine effects in human volunteers. J Pharmacol Exp Ther 264:813–823
Preston KL, Jasinski DR, Testa M (1991) Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend 27:7–17
Radbruch L, Grond S, Lehmann KA (1996) A risk–benefit assessment of tramadol in the management of pain. Drug Saf 15:8–29
Raffa RB (2008) Basic pharmacology relevant to drug abuse assessment: tramadol as example. J Clin Pharm Ther 33:101–108
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL (1992) Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260:275–285
Ren YH, Zheng JW (2000) Influence of tramadol on morphine discriminative behavior in rats. Acta Pharmacol Sin 21:924–926
Rouini MR, Ardakani YH, Soltani F, Aboul-Enein HY, Foroumadi A (2006) Development and validation of a rapid HPLC method for simultaneous determination of tramadol and its two main metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 830:207–211
Schneider MF, Bailey JE, Cicero TJ, Dart RC, Inciardi JA, Parrino M, Muñoz A (2009) Integrating nine prescription opioid analgesics and/or four signal detection systems to summarize statewide prescription drug abuse in the United States in 2007. Pharmacoepidemiol Drug Saf 18:778–790
Schuh KJ, Walsh SL, Bigelow GE, Preston KL, Stitzer ML (1996) Buprenorphine, morphine and naloxone effects during ascending morphine maintenance in humans. J Pharmacol Exp Ther 278:836–846
Schuh KJ, Walsh SL, Stitzer ML (1999) Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology (Berl) 145:162–174
Sprague JE, Leifheit M, Selken J, Milks MM, Kinder DH, Nichols DE (2002) In vivo microdialysis and conditioned place preference studies in rats are consistent with abuse potential of tramadol. Synapse 43:118–121
Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F (2007) Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther 82:41–47
Stoops WW, Hatton KW, Lofwall MR, Nuzzo PA, Walsh SL (2010) Intravenous oxycodone, hydrocodone and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology (Berl) 212:193–203
Substance Abuse and Mental Health Services Administration, Office of Applied Studies (2007) The NSDUH report: patterns and trends in nonmedical prescription pain reliever use: 2002 to 2005. SAMHSA, Rockville, MD
Substance Abuse and Mental Health Services Administration, Office of Applied Studies (2009) The NSDUH report: trends in nonmedical use of prescription pain relievers: 2002 to 2007. SAMHSA, Rockville, MD
Substance Abuse and Mental Health Services Administration (2011) Results from the 2010 national survey on drug use and health: summary of national findings. NSDUH Series H-41, HHS publication no. (SMA) 11-4658. SAMHSA, Rockville, MD
Sullivan MA, Vosburg SK, Comer SD (2006) Depot naltrexone: antagonism of the reinforcing, subjective and physiological effects of heroin. Psychopharmacology 189:37–46
Swedberg MD, Shannon HE, Nickel B, Goldberg SR (1988) Pharmacological mechanisms of action of flupirtine: a novel, centrally acting, nonopioid analgesic evaluated by its discriminative effects in the rat. J Pharmacol Exp Ther 246:1067–1074
Swedberg MD, Shannon HE, Nickel B, Goldberg SR (1992) D-16949 (anpirtoline): a novel serotonergic (5-HT1B) psychotherapeutic agent assessed by its discriminative effects in the rat. J Pharmacol Exp Ther 263:1015–1022
Takemori AE, Ho BY, Naeseth JS, Portoghese PS (1988) Nor-Binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther 246:255–258
Tzschentke TM, Bruckmann W, Friderichs E (2002) Lack of sensitization during place conditioning in rats is consistent with the low abuse potential of tramadol. Neurosci Lett 329:25–28
Vanderkooy JD, Kennedy SH, Bagby RM (2002) Antidepressant side effects in depression patients treated in a naturalistic setting: a study of bupropion, moclobemide, paroxetine, sertraline and venlafaxine. Can J Psychiatry 47:174–180
Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830
Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL (2011) Uniform assessment and ranking of Mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol 59:385–390
Walsh SL, Preston KL, Bigelow GE, Stitzer ML (1995) Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther 274:361–372
Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE (1996) Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther 279:524–538
Walsh SL, Chausmer AE, Strain EC, Bigelow GE (2008a) Evaluation of the mu and kappa opioid actions of butorphanol in humans through differential naltrexone blockade. Psychopharmacology (Berl) 196:143–155
Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR Jr (2008b) The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend 98:191–202
Yanagita T (1978) Drug dependence potential of 1-(m-methoxyphenyl)-2-dimethylaminomethyl)-cyclohexan-1-ol hydrochloride (tramadol) tested in monkeys. Arzneimittelforschung 28:158–163
Yen TT, Fuller RW (1992) Preclinical pharmacology of fluoxetine, a serotonergic drug for weight loss. Am J Clin Nutr 55(1 Suppl):177S–180S
Zacny JP (2005) Profiling the subjective, psychomotor and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend 80:273–278
Acknowledgments
This research was supported by grant number R01DA025649 from the National Institute on Drug Abuse (PI: William W. Stoops) and by grant number UL1RR033173 from the National Center for Research Resources (PI: Philip A. Kern). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, NCRR, or NIH. The authors declare no conflicts of interest relevant to this project. The authors wish to thank the staff at the University of Kentucky Center on Drug and Alcohol Research for technical and medical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1470 kb)
Rights and permissions
About this article
Cite this article
Stoops, W.W., Lofwall, M.R., Nuzzo, P.A. et al. Pharmacodynamic profile of tramadol in humans: influence of naltrexone pretreatment. Psychopharmacology 223, 427–438 (2012). https://doi.org/10.1007/s00213-012-2739-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2739-4