Abstract
Rational
The ghrelinergic system is implicated in the development of obesity and in modulating central reward systems. It has been reported that diet-induced obesity causes blunted responding on food intake to ghrelin administration, associated with central ghrelin resistance. Here we investigate whether the stimulatory effects of ghrelin on the reward system are altered in diet-induced obese mice.
Methods
Obesity was induced in C57BL/6J mice by feeding high-fat diet for 13 weeks. Mice were trained in an operant fixed and exponential progressive ratio task to respond for sucrose rewards. In an ad libitum fed state, ghrelin and a ghrelin receptor antagonist were administered in the progressive ratio. Alterations in the central ghrelin system in diet-induced obese mice were assessed.
Results
Obese mice showed attenuated acquisition and performance in the fixed and progressive ratio paradigm. Most importantly, diet-induced obesity inhibited the stimulatory effects of ghrelin (2 nmol, 3 nmol/10 g) on progressive ratio responding whereas lean animals presented with increased responding. Administration of the ghrelin-receptor antagonist (D-Lys3)-GHRP-6 (66.6 nmol/10 g) decreased performance in lean but not obese mice. This insensitivity to ghrelin receptor ligands in mice on high-fat diet was further supported by decreased mRNA expression of the ghrelin receptor in the hypothalamus and the nucleus accumbens in obese mice.
Conclusions
This study demonstrates that the modulatory effects of ghrelin receptor ligands are blunted in a mouse model of diet-induced obesity in a progressive ratio task. Thereby, our data extend the previously described ghrelin resistance in these mice from food intake to reward-associated behaviours.
Similar content being viewed by others
Introduction
The role of the ghrelinergic system in regulating energy homeostasis and modulating food intake and eating behaviour has been well-characterised (for review, see Cheng et al. 2010; Schellekens et al. 2010). More recently, several studies have demonstrated the importance of ghrelin and the ghrelin receptor in reward-associated circuits (Abizaid et al. 2006; Jerlhag et al. 2006; and for review, see Dickson et al. 2011). Administration of ghrelin increases drug- and food-seeking behaviours, and augments the rewarding aspects of sucrose, alcohol and high-fat diet in rodents (Landgren et al. 2011a, b; Perello et al. 2010). Pharmacological antagonism of the ghrelin receptor on the other hand inhibits the rewarding aspects of drugs, as seen in decreased responding for cocaine, amphetamine, nicotine or alcohol administration (Jerlhag et al. 2009, 2010; Jerlhag and Engel 2011). Furthermore, cocaine-seeking is associated with elevated plasma levels of ghrelin and peripheral administration of ghrelin enhances cocaine-induced locomotor stimulation and conditioned place preference in rodents (Davis et al. 2007; Tessari et al. 2007; Wellman et al. 2005). This implicates ghrelin as an activator of reward pathways, including dopaminergic circuits from the ventral tegmental area to the nucleus accumbens (for review, see Dickson et al. 2011). Alterations in these pathways are of clinical importance, as they can be observed in drug addictions as well as eating disorders (Kenny 2011; Volkow et al. 2008). In obesity, changes in food intake and reward-associated behaviour can be observed, often accompanied by alterations in eating patterns and increased intake of foods with high fat and sugar content (for review, see Vucetic and Reyes 2010). Furthermore, studies in humans and animals have shown a dysregulation of the ghrelinergic system in obesity (for review, see Yi et al. 2011).
Of note, as recently shown (Briggs et al. 2010), the long-term exposure to high-fat diet in the diet-induced obese mouse model leads to a suppression of the ghrelinergic system with decreased hypothalamic expression of the ghrelin receptor and decreased peripheral ghrelin levels and ghrelin expression in the stomach. Moreover, this study demonstrated that administration of ghrelin failed to increase food intake in diet-induced obese mice and did not promote hypothalamic mRNA expression of NPY and AgRP. Taken together, these results indicate central ghrelin resistance in diet-induced obese mice (Briggs and Andrews 2011; Briggs et al. 2010). However, the potential for generalisation of these findings to other feeding-associated paradigms has not been well investigated to date.
Changes in the reward system play an important role in obesity (Volkow and O’Brien 2007; Volkow and Wise 2005; Vucetic and Reyes 2010; Wang et al. 2001), and alterations in the functionality of the ghrelinergic system have been associated with an obese state (Yildiz et al. 2004). The progressive ratio is an important tool to study responding for addictive drugs and natural rewards in animals (Richardson and Roberts 1996; Sabino et al. 2011; Skibicka et al. 2011b) and humans (Sofuoglu et al. 2011; Stoops 2008; Stoops et al. 2010; Temple et al. 2008). Surprisingly, this paradigm has rarely been used in obesity research in mice. However, we have recently demonstrated the utility of an exponential progressive ratio tasks in leptin-deficient obese mice (Finger et al. 2010) to investigate reward-associated behaviours in obesity.
There is a paucity of studies investigating convergence of the described blunted sensitivity to ghrelin stimulation in diet-induced obesity with ghrelin’s function in stimulating rewarded behaviours. Therefore, in this study, we test the hypothesis that central ghrelin resistance in diet-induced obese animals inhibits the reward-associated aspects of ghrelin in progressive ratio responding in mice. Animals were trained in a recently validated operant task (Finger et al. 2010) to assess baseline differences in acquisition and fixed and progressive ratio responding. Ghrelin and (D-Lys3)-GHRP-6, respectively, were administered peripherally to assess the impact of ghrelin insensitivity on responding in an exponential progressive ratio paradigm in this mouse model. To further investigate possible changes in the ghrelinergic system in diet-induced obese mice, central mRNA expression levels of ghrelin and the ghrelin receptor GHSR1a were assessed.
Materials and methods
Animals
In this study, male C57BL/6J mice (n = 24; operant testing group and n = 16; naïve sample group; Harlan, UK) were used. After arrival, the group-housed 3-week-old C57BL/6J mice received high-fat (45% kcal from fat (lard 87.7%, soybean oil 12.3%); Research Diets; #D12451, New Brunswick, NJ) or low-fat diet (10%kcal from fat (soybean oil56%, lard 44%), Research Diets, New Brunswick, NJ; #D12450B), respectively, throughout the whole study. As described previously (Finger et al. 2011b), feeding was carried out for 13 weeks to induce adiposity in the obesity prone C57BL/6J strain (Takahashi et al. 2002). Water and food were available ad libitum to all mice throughout the whole study unless stated otherwise. The holding room was temperature (21 ± 1°C) and humidity (55 ± 10%) controlled and under a 12-h light/dark cycle (lights on 5:00 a.m.).
All experiments were conducted in accordance with the European Directive 86/609/EEC, the Recommendation 2007/526/65/EC and approved by the Animal Experimentation Ethics Committee of University College Cork. All efforts were made to minimise animal sufferings and to reduce the number of animals used.
Apparatus
Operant testing started after 13 weeks of feeding. Experiments were performed in eight standard mouse operant chambers (MED-307A-B2; Med Associates Inc., St. Albans, VT) described previously (Finger et al. 2010). Briefly, chambers (15.9 × 14.0 × 12.7 cm interior dimensions) were located in a sound attenuating cubicle and equipped with one illuminated nosepoke response hole and a food magazine both containing ultra-sensitive photo beams. Responding at the nosepoke hole was reinforced by the delivery of a 20-mg peanut-butter-flavoured sucrose pellet (Test Diet, Richmond, IN) from a 20 mg-pellet dispenser (Med Associates). All test chambers were controlled by a PC using Med-PC software.
Experimental design and drugs
All drugs were administrated in an ad libitum feeding state with 4 days of washout between drug administrations in crossover designs. Rat ghrelin (Innovagen, Sweden) and the ghrelin receptor antagonist (D-Lys3)-GHRP-6 (Bachem, Ireland) were freshly dissolved in sterile, cooled 0.9% saline and kept on ice between injections. (D-Lys3)-GHRP-6 is a widely in vivo and in vitro studied and applied ghrelin-receptor antagonist (Beck et al. 2004; Kitazawa et al. 2005), with strong antagonistic activity the GHSR1a and a low affinity at melanocortin receptors (Schiöth et al. 1997). Peripheral administration of the antagonist (D-Lys3)-GHRP-6 has previously been shown to have similar effects compared to central administration (Asakawa et al. 2003) via its activity at ghrelin receptors in the arcuate nucleus, where the blood brain barrier is incomplete (Pulman et al. 2006). Furthermore, peripheral and central ghrelin administration triggers an increase in responding to rewards in operant tasks, as shown by Skibicka et al. (2011b). Both drugs and vehicle (sterile saline) were administered intraperitoneal (i.p.) immediately prior to progressive ratio (PR) testing in concentrations of 2 and 3 nmol/10 g [ghrelin] of body weight and 66.6 nmol/10 g [(D-Lys3)-GHRP-6], as previously described (Finger et al. 2011c). Timepoints of administration were chosen based on cumulative food intake studies applying ghrelin and the ghrelin-receptor antagonist (D-Lys3)-GHRP-6 (Finger et al. 2011c).
Experimental procedures
As we have shown previously (Finger et al. 2011c), the time of day significantly affects feeding behaviour, and testing in the dark phase increases baseline intake. Therefore, testing started 1 h after the onset of darkness.
Habituation
Mice were habituated to the operant chamber and the reward pellets for 3 days prior to testing. Unconditioned magazine training was conducted on two consecutive days. Animals were placed into the operant chamber for 15 min twice per day with reward pellets freely available in the magazine. The nosepoke response hole was closed with adhesive tape during habituation and the following non-contingent training. Only when all mice collected the reward from the magazine, conditioned magazine training was started.
Conditioned magazine training
Animals received two 15-min sessions per day on two consecutive days with reward delivery that was non-contingent to nosepoke responding. This conditioned magazine training was carried out to ensure that all mice were familiar with reward collection from the magazine. Therefore, each animal was placed into the assigned operant chamber and then had to initiate the trial by entering the food magazine with the head. After 30 s, one reward pellet was delivered into the magazine. A new pellet was only given if the mouse had entered the food magazine after reward delivery. Only when all mice were entering the food magazine freely to collect the food reward, fixed ratio testing was carried out.
All operant programmes used were adapted from previous work (Finger et al. 2010) for testing in the dark phase; therefore, the house light was permanently turned off.
Fixed ratio
For fixed ratio training and testing mice were mildly food restricted to initiate responding and facilitate learning. Therefore, food was removed from the cages in the morning and replaced after all animals had performed the task. Mice received 3 days with one 15-min training session in the fixed ratio schedule 1 (FR1), where the nosepoke hole was primed at the start of the session with a small diet pellet to initiate and increase nosepoking. Animals were placed into the chamber and had to enter the magazine to initiate the trial. Then, the light (conditioned stimulus (CS) and discriminative stimulus) inside the nosepoke hole was turned on awaiting a response. One nosepoke then triggered the delivery of one reward pellet (unconditioned stimulus (US)), the nosepoke light was turned off and an entry into the magazine was required. Further, nosepoke responding without previous entry into the magazine did not trigger the delivery of another reward. Within the three primed FR1 sessions, all animals formed the CS–US association.
From the next day on, mice received one daily 30-min FR1 sessions using the programme described above, but without priming of the nosepoke hole. Mice were trained in the FR1 setting for three consecutive days. Subsequently, response demands were increased to FR3 and FR6. Again, mice received one daily session for three consecutive days in each training stage.
Exponential progressive ratio and the effects of ghrelin receptor ligands
Following fixed ratio testing, mice were trained in an exponential PR schedule as described previously (Finger et al. 2010). Response demands increased according to r = (5 × e0.2n)−5, rounded to the nearest integer and with n as the position in the sequence of ratios (Mobini et al. 2000; Richardson and Roberts 1996; Rickard et al. 2009). Animals had a time window of 15 min to complete each level of the PR. Upon failure to fulfil this requirement, the programme stopped (time out), and mice were immediately removed from the operant chamber. The last level completed in the PR before time out was considered as the breakpoint. For the first 4 days of PR testing, mice remained on the mild restriction schedule described above. Subsequently to prepare for drug administration sessions in an ad libitum state, mice were maintained on free feeding and left undisturbed for 3 days until the body weight loss of approximately 1 to 2 g from mild food restriction was back to free feeding levels in all animals. Animals were then tested in the PR programme for baseline ad libitum performance for 4 days prior to the first drug administration.
Subsequently, changes in PR responding were assessed following administration of ghrelin (3 nmol/10 g) and the ghrelin receptor antagonist (D-Lys3)-GHRP-6 (66.6 nmol/10 g). Between drug sessions, animals received 4 days of washout with baseline PR testing.
Central mRNA expression of ghrelin and the ghrelin receptor GHSR1a
Animals from the independent naïve sample group (n = 8/diet) were left undisturbed for 15 weeks of ad libitum feeding of high- and low-fat diet. Mice were then sacrificed without anaesthesia, the brains removed, hypothalamus and nucleus accumbens dissected and stored in 300 μl RNAlater® at 4°C for 24 h, followed by freezing at −80°C. Tissue samples of the nucleus accumbens were pooled from both hemispheres. Total RNA isolation, quantification and reverse transcription were carried out as previously described (Finger et al. 2011c). Real-time quantitative RT-PCR was performed using mouse-specific Ghrl and GHSR1a probes (Applied Biosystems, Warrington, UK). All samples were run in triplicate, and values were expressed as fold-change normalized to the low-fat diet group as described previously (Finger et al. 2011c).
Statistical analysis
Statistical analysis of the fixed ratio was performed using two-phase repeated-measures ANOVA, and of the exponential progressive ratio using repeated measures ANOVA; both followed by estimation of parameters. The effects of ghrelin and the ghrelin receptor antagonist on PR responding were analysed using two-way ANOVA. Differences between individual groups were assessed using protected LSD post hoc tests, but only following significant overall effects, as previously described (Finger et al. 2011c; Jerlhag et al. 2008). Central mRNA expression levels were analysed using independent sample t tests. All tests were carried out at a significance level of p < 0.05. All analysis was carried out using SPSS 15.0 for windows (SPSS Inc., Chicago, IL). All graphs show mean values ± SEM.
Results
Body weight
Over the 13 weeks of feeding, mice on a high-fat diet gained significantly more body weight than mice on low-fat diet (time × diet: F(5,110) = 11.848; p < 0.001; diet F(1,22) = 84.446; p < 0.001; Fig. 1). At the start of operant training mice in the high-fat diet group weighed 42.1 g ± 4.2 and 33.6 g ± 2.6 in the low-fat diet group.
Diet-induced obese mice show decreased fixed ratio acquisition and responding
Repeated-measures analysis of the number of responses in the fixed ratio paradigm was analysed for FR1, FR3 and FR6 (three sessions in each demand). Across all three fixed ratio stages of different demand (1, 3 and 6) mice on a high-fat diet responded less and showed less improvement in performance compared to mice on a low-fat diet (demand × session × diet: F(4,88) = 9.254; p < 0.001; diet: F(1,22) = 33.968; p < 0.001; Fig. 2). Within the FR1 stage, the low-fat diet group increased responding over sessions, whereas this was not observed in the high-fat diet group (session × diet: F(2,44) = 14.867; p < 0.001; diet F(1,22) = 17.692; p < 0.001; Fig. 2), showing low acquisition learning. At the FR3 stage, high-fat diet mice again responded less compared to low-fat diet mice (diet: F(1,22) = 32.263; p < 0.001). Over sessions, both groups improved performance, although the pattern and extent of improvement varied (session × diet F(2,44) = 9.599; p < 0.001, Fig. 2). Similar results were observed in FR6, with lower responding and improvement in the high-fat diet group (session × diet: F(2,44) = 5.634; p = 0.007; diet: F(1,22) = 31.910; p < 0.001; Fig. 2).
Diet-induced obesity decreases responding in the progressive ratio
Across the four baseline sessions of exponential progressive ratio testing under mild food restrictions (Fig. 3a), mice on a high-fat diet reached lower breakpoints (the last levels completed before time-out) than mice on a low-fat diet (diet: F(1,22) = 16.386; p = 0.001). In an ad libitum state (Fig. 3b), baseline testing showed a similar profile between groups (diet: F(1,22) = 7.814; p = 0.011).
Mice on high-fat diet had decreased responding in the exponential PR under baseline conditions with mild food restriction (a) and under ad libitum feeding (b). Performance was rated according to the last PR ratio completed (breakpoint) within the 15 min time interval before time out. LFD low-fat diet; HFD high-fat diet; *p < 0.05; **p < 0.01; ***p < 0.001; n = 12/diet group
Diet-induced obesity causes insensitivity to ghrelin receptor ligands—drug-induced changes in progressive ratio responding
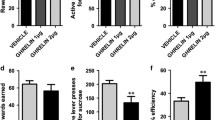
In an ad libitum state, administration of ghrelin (2 and 3 nmol/10 g body weight) and of the ghrelin receptor antagonist (D-Lys3)-GHRP-6 (66.6 nmol/10 g body weight) altered responding in the progressive ratio paradigm only in mice on a low-fat diet (diet × drug F(3.84) = 2.801; p = 0.045; diet F(1,84) = 24.269; p < 0.001; drug F(3,84) = 6.048; p = 0.001; Fig. 4). Ghrelin increased breakpoint levels in mice receiving low-fat diet only at a dose of 3 nmol/10 g (ghrelin vs. vehicle p = 0.011) but not at a dose of 2 nmol/10 g (p = 0.301). However, in mice on high-fat diet, administration of ghrelin did not change breakpoint levels at both doses (p = 0.932, 3 nmol/10 g and p = 0.842, 2 nmol/10 g vs. vehicle).
Diet-induced obese mice were insensitive to the ghrelin receptor ligands in the progressive ratio. Ghrelin (2 and 3 nmol/10 g bodyweight; i.p.) increased responding only in mice on low-fat diet at the higher dose. Similarly, the ghrelin-receptor antagonist (D-Lys3)-GHRP-6 (66.6 nmol/10 g bodyweight; i.p.) decreased responding only in mice on low-fat diet. LFD Low-fat diet, HFD high-fat diet; (D-Lys3)-GHRP-6; *p < 0.05; n = 12 in all HFD-groups, n = 11 in all LFD groups
Similarly, sensitivity to the anorexigenic ghrelin receptor antagonist led to a reduction in responding and thereby lower breakpoint levels in mice on low-fat diet (p = 0.038 vs. vehicle), whereas mice on a high-fat diet showed blunted sensitivity to the antagonist (p = 0.321 vs. vehicle). Responding under vehicle conditions was marginal significant between diet groups (p = 0.069), comparable to sessions 3 and 4 under baseline conditions (Fig. 3b). One animal of the low-fat diet group did not participate in all drug sessions due to an idiosyncratic reaction to the injection procedure.
Central mRNA expression of ghrelin and the ghrelin receptor GHSR1a
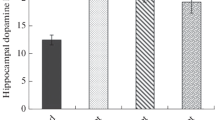
In the independent experimentally naïve sample group (n = 8/diet) hypothalamic mRNA expression (Fig. 5a) of both, ghrelin (t = 2.422; df = 14; p = 0.030), and the ghrelin receptor GHSR1a (t = 2.209; df = 14; p = 0.044) was decrease in diet-induced obese mice compared to lean animals (Fig. 5a). Similarly, mRNA expression of the GHSR1a in was decreased in the nucleus accumbens (Fig. 5b) in obese compared to lean mice (t = 2.232; df = 14; p = 0.042).
Hypothalamic mRNA expression (a) of ghrelin and the ghrelin receptor GHSR1a was significantly reduced in experimentally naïve diet-induced obese mice compared to mice on a low-fat diet. In the nucleus accumbens (b), mRNA expression of the GHSR1a was significantly decreased in a state of diet-induced obesity. LFD low-fat diet, HFD high-fat diet; *p < 0.05; n = 8/diet group
Discussion
In this study, we demonstrate that diet-induced central ghrelin resistance (Briggs et al. 2010) extends beyond the previously described food intake to rewarded behaviour in a complex task. In the progressive ratio, we demonstrate that the modulatory effects of ghrelin receptor ligands on responding are blunted in diet-induced obese mice, whereas in lean animals, ghrelin and the ghrelin receptor antagonist (D-Lys3)-GHRP-6 increase and decrease progressive ratio responding, respectively.
Fixed and progressive ratio schedules of operant responding are well-utilized tasks to assess the rewarding aspects and reinforcing potency of addictive drugs or natural rewards in animals and humans (Haney et al. 2011; Richardson and Roberts 1996; Roane 2008; Sofuoglu et al. 2011; Stoops et al. 2010; Vlachou and Markou 2010). Whereas in a fixed ratio schedule the respond-demand increases between sessions, it increases in progressive ratio tasks within a session, often in an exponential fashion (Arnold and Roberts 1997). This allows testing the subject’s motivation to obtain a reward in a “cost–benefit” approach (El-Ghundi et al. 2003; Hodos 1961). Interestingly, progressive ratio tasks have only recently been applied to mouse model of obesity (Finger et al. 2010). Here, we show that the task can also be used in diet-induced obesity models.
The role of the ghrelinergic system in reward is a newly emerging field. Several recent studies have demonstrated that administration of ghrelin increases drug and food seeking behaviour and enhances the rewarding aspects of high-fat diet (Landgren et al. 2011a, b; Perello et al. 2010). In a progressive ratio task in rats, ghrelin increases sucrose self-administration and activates the main central reward pathways, whereas antagonism at the ghrelin receptor inhibits reward seeking for palatable food (Egecioglu et al. 2010; Landgren et al. 2011b; Skibicka et al. 2011a, b). Furthermore, the role of the ghrelinergic system in obesity is a promising target in today’s development of anti-obesity drugs (Field et al. 2009). Several studies in humans have implicated that alterations in these reward circuits underlie changes in eating behaviour linked to obesity, with an overconsumption of sweet and fat foods (Yi et al. 2011). Although the strong link between ghrelin, food intake, reward, motivation and obesity is well known (for review, see Dickson et al. 2011; Egecioglu et al. 2011; Lutter and Nestler 2009), there is a paucity of studies investigating the inter-relationship of these factors with one another within one experimental design.
In the first part of the study, we show that mice after long-term exposure to high-fat diet show decreased acquisition and delayed improvement in the fixed ratio paradigm. These findings are in line with one previous study in rats demonstrating that diet-induced obese rats, in both an ad libitum and pair fed state, display attenuated performance in fixed and progressive ratio operant schedules responding for sucrose reward (Davis et al. 2008). This has been linked to a decreased dopamine turnover within mesolimbic reward-associated areas or increased circulating levels of leptin (Davis et al. 2008). The fact that these findings are reproducible between species supports the validity of the widely used model of high-fat diet-induced obesity in rats as well as in mice. However, most of the operant-based paradigms in the literature are carried out in rats, as they are easier to train and show superior performance in operant tasks. Our study proves that the mouse even in a state of obesity and at 16 weeks of age at the beginning of training can more than adequately perform these tasks. This is especially important, as mouse models of obesity have become widely used in obesity research (Kirchner et al. 2010; Tschöp and Heiman 2001).
In the exponential progressive ratio task, we then investigated the effects of ghrelin on rewarded responding. Interestingly, following administration of ghrelin (2 and 3 nmol/10 g) in an ad libitum fed state, obese mice showed no change in responding for sucrose rewards. These data confirm that the previously described ghrelin resistance in this mouse model as assessed by blunted food intake following ghrelin administration (Briggs and Andrews 2011; Briggs et al. 2010) generalises to ghrelin’s effects in a rewarded task. Moreover, proving the validity of the progressive ratio task and confirming the previously described results in normal fed rats (Skibicka et al. 2011a, b), our low-fat diet mice showed a significant increase in responding after ghrelin administration. Thus, these data suggest that the neurobiology underlying obesity-induced ghrelin resistance and ghrelin’s role in rewarded behaviour are overlapping.
We then tested whether antagonism at the ghrelin receptor can reduce progressive ratio responding in an ad libitum state. Again, confirming our findings on the effects of ghrelin in mice on low- and high-fat diet, only lean mice showed significantly decreased responding following administration of the ghrelin receptor antagonist (D-Lys3)-GHRP-6. These results in lean animals are in line with studies on ghrelin receptor antagonism in non-obese rodents, describing decreased responding to the rewarding aspects of food rewards, cocaine, amphetamine, nicotine and alcohol (Egecioglu et al. 2010; Jerlhag et al. 2009, 2010; Jerlhag and Engel 2011; Landgren et al. 2011a). Most importantly, diet-induced obese mice, however, did not show this reduction in responding, re-confirming the decreased sensitivity of the ghrelinergic system in high-fat diet-induced obese animals. These diet-induced differences are intriguing, and future studies should therefore investigate a full dose–response profile of ghrelin and the ghrelin-receptor antagonist in rewarded operant behaviour in mouse models of diet-induced obesity.
These data were further supported by decreased mRNA expression levels of ghrelin and the ghrelin receptor GHSR1a in the hypothalamus in a separate experimentally naïve group of obese mice compared to control fed animals. These data are in line with those described previously by Briggs et al. (2010). These authors also demonstrated that this decrease at the hypothalamic level together with a reduction in circulating peripheral ghrelin levels in obese mice suggests an overall desensitisation of the central ghrelin system in diet-induced obesity. This concept is supported by our results on the blunted responding of obese mice to ghrelin receptor ligands in the progressive ratio task. We further investigated expression of the ghrelin receptor in an important area of the reward system, the nucleus accumbens. Here, similar to the hypothalamus, obese mice showed a decrease in expression supporting our findings in the progressive ratio. Intriguingly, Skibicka and colleagues recently demonstrated (2011a) that expression of the GHSR1a is significantly higher in the ventral tegmental area than in the nucleus accumbens and that local infusion of ghrelin predominantly stimulates sucrose intake via the ventral tegmental area. Further studies should therefore investigate the expression profile of the GHSR1a in the ventral tegmental area in diet-induced obese animals.
Moreover, our behavioural results point out interesting differences between mouse models of obesity in regards to appetitive operant responding as well as sensitivity towards ghrelinergic ligands. We have shown previously that leptin-deficient ob/ob mice display no difference in fixed ratio tasks of lower demand as well as in a linear and exponential progressive ratio schedule (Finger et al. 2010). We furthermore showed that the leptin-deficient mouse is sensitive to ghrelinergic ligands in a cumulative food intake tasks. However, sensitivity was dependent on the time of day and the state of satiety (Finger et al. 2011c). In the behavioural satiety sequence, a task of fine-structured analysis of feeding and satiety (Rodgers et al. 2010) showed less changes in the satiety profile in ob/ob mice compared to lean control animals (Finger et al. 2011a).
In conclusion, this study further confirms the utility of mouse-based operant paradigms in the obesity and reward field and illustrates how they can be applied to a diet-induced mouse model of obesity. We verified this aspect by convincingly reproducing the previously in rats described attenuation of responding for sucrose rewards in high-fat diet-induced obese animals. We demonstrate that ghrelin has the ability to increase responding for rewards whereas a ghrelin receptor antagonist blocks such responding. Most importantly, we confirmed that the previously blunted effects of ghrelin on food intake induced by long-term consumption of high-fat diet (Briggs et al. 2010) also translate to a progressive ratio-operant design. Herein, we proved that this resistance inhibits the modulating effects of ghrelin receptor ligands on progressive ratio responding in diet-induced obese mice, further confirming ghrelin’s role at the interface of obesity and reward.
References
Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239
Arnold JM, Roberts DC (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57:441–447
Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M (2003) Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52:947–952
Beck B, Richy S, Stricker-Krongrad A (2004) Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci 76:473–478
Briggs DI, Andrews ZB (2011) Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology 93:48–57
Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB (2010) Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151:4745–4755
Cheng KC, Li YX, Asakawa A, Inui A (2010) The role of ghrelin in energy homeostasis and its potential clinical relevance. Int J Mol Med 26:771–778
Davis KW, Wellman PJ, Clifford PS (2007) Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept 140:148–152
Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, Benoit SC (2008) Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 122:1257–1263
Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (2011) The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 340(1):80–87
Egecioglu E, Jerlhag E, Salomé N, Skibicka KP, Haage D, Bohlooly-Y M, Andersson D, Bjursell M, Perrissoud D, Engel JA, Dickson SL (2010) Ghrelin increases intake of rewarding food in rodents. Addict Biol 15:304–311
Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Jerlhag E, Engel JA, Dickson SL (2011) Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord 12(3):141–151
El-Ghundi M, O’Dowd BF, Erclik M, George SR (2003) Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci 17:851–862
Field BC, Chaudhri OB, Bloom SR (2009) Obesity treatment: novel peripheral targets. Br J Clin Pharmacol 68:830–843
Finger BC, Dinan TG, Cryan JF (2010) Progressive Ratio Responding in an Obese Mouse Model: Effects of Fenfluramine. Neuropharmacology 59:619–626
Finger BC, Dinan TG, Cryan JF (2011a) Behavioural satiety sequence in a genetic mouse model of obesity: effects of ghrelin receptor ligands. Behav Pharmacol. doi:10.1097/FBP.0b013e32834afee6
Finger BC, Dinan TG, Cryan JF (2011b) High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. doi:10.1016/j.neuroscience.2011.06.072
Finger BC, Schellekens H, Dinan TG, Cryan JF (2011c) Is there altered sensitivity to ghrelin receptor ligands in leptin deficient mice?: Importance of satiety state and time of day. Psychopharmacology (Berl) 216:421–429
Haney M, Rubin E, Foltin RW (2011) Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology (Berl) 216(3):379–387
Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134:943–944
Jerlhag E, Engel JA (2011) Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend 117(2–3):126–131
Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA (2006) Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 11:45–54
Jerlhag E, Egecioglu E, Dickson SL, Svensson L, Engel JA (2008) Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors are involved in mediating the ghrelin-induced locomotor stimulation and dopamine overflow in nucleus accumbens. Eur Neuropsychopharmacol 18:508–518
Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA 106:11318–11323
Jerlhag E, Egecioglu E, Dickson SL, Engel JA (2010) Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 211:415–422
Kenny PJ (2011) Reward mechanisms in obesity: new insights and future directions. Neuron 69:664–679
Kirchner H, Tong J, Tschöp MH, Pfluger PT (2010) Ghrelin and PYY in the regulation of energy balance and metabolism: lessons from mouse mutants. Am J Physiol Endocrinol Metab 298:E909–E919
Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL (2005) Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut 54:1078–1084
Landgren S, Simms JA, Hyytiä P, Engel JA, Bartlett SE, Jerlhag E (2011a) Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats. Addict Biol. doi:10.1111/j.1369-1600.2010.00280.x
Landgren S, Simms JA, Thelle DS, Strandhagen E, Bartlett SE, Engel JA, Jerlhag E (2011b) The ghrelin signalling system is involved in the consumption of sweets. PLoS One 6:e18170
Lutter M, Nestler EJ (2009) Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139:629–632
Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E (2000) Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology (Berl) 152:47–54
Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM (2010) Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry 67:880–886
Pulman KJ, Fry WM, Cottrell GT, Ferguson AV (2006) The subfornical organ: a central target for circulating feeding signals. J Neurosci 26:2022–2030
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Rickard JF, Body S, Zhang Z, Bradshaw CM, Szabadi E (2009) Effect of reinforcer magnitude on performance maintained by progressive-ratio schedules. J Exp Anal Behav 91:75–87
Roane HS (2008) On the applied use of progressive-ratio schedules of reinforcement. J Appl Behav Anal 41:155–161
Rodgers RJ, Holch P, Tallett AJ (2010) Behavioural satiety sequence (BSS): Separating wheat from chaff in the behavioural pharmacology of appetite. Pharmacol Biochem Behav 97:3–14
Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP (2011) Activation of σ-Receptors Induces Binge-like Drinking in Sardinian Alcohol-Preferring Rats. Neuropsychopharmacology 36:1207–1218
Schellekens H, Dinan TG, Cryan JF (2010) Lean mean fat reducing “ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Review. Neuropharmacology 58:2–16
Schiöth HB, Muceniece R, Wikberg JE (1997) Characterization of the binding of MSH-B, HB-228, GHRP-6 and 153N-6 to the human melanocortin receptor subtypes. Neuropeptides 31:565–571
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL (2011a) Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180:129–137
Skibicka KP, Hansson C, Egecioglu E, Dickson SL (2011b) Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. doi:10.1111/j.1369-1600.2010.00294.x
Sofuoglu M, Mooney M, Kosten T, Waters A, Hashimoto K (2011) Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology (Berl) 213:61–68
Stoops WW (2008) Reinforcing effects of stimulants in humans: sensitivity of progressive-ratio schedules. Exp Clin Psychopharmacol 16:503–512
Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR (2010) Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur J Pharmacol 644:101–105
Takahashi N, Patel HR, Qi Y, Dushay J, Ahima RS (2002) Divergent effects of leptin in mice susceptible or resistant to obesity. Horm Metab Res 34:691–697
Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH (2008) Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr 87:1121–1127
Tessari M, Catalano A, Pellitteri M, Di Francesco C, Marini F, Gerrard PA, Heidbreder CA, Melotto S (2007) Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addict Biol 12:22–29
Tschöp M, Heiman ML (2001) Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109:307–319
Vlachou S, Markou A (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371
Volkow ND, O’Brien CP (2007) Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry 164:708–710
Volkow ND, Wise RA (2005) How can drug addiction help us understand obesity? Nat Neurosci 8:555–560
Volkow ND, Wang GJ, Fowler JS, Telang F (2008) Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 363:3191–3200
Vucetic Z, Reyes TM (2010) Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med 2:577–593
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS (2001) Brain dopamine and obesity. Lancet 357:354–357
Wellman PJ, Davis KW, Nation JR (2005) Augmentation of cocaine hyperactivity in rats by systemic ghrelin. Regul Pept 125:151–154
Yi CX, Heppner K, Tschöp MH (2011) Ghrelin in eating disorders. Mol Cell Endocrinol 340(1):29–34
Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J (2004) Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 101:10434–10439
Acknowledgments
The work described herein was supported by Enterprise Ireland under Grant Number CC20080001. JFC and TGD are also supported in part by Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre). The centre is also funded by GlaxoSmithKline. JFC is funded by European Community’s Seventh Framework Programme; Grant Number: FP7/2007-2013, Grant Agreement 201714.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finger, B.C., Dinan, T.G. & Cryan, J.F. Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology 220, 173–181 (2012). https://doi.org/10.1007/s00213-011-2468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2468-0